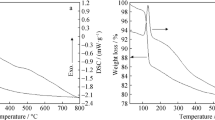
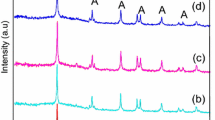
Abstract
In this paper, Cu-doped TiO2 powder was prepared by sol–gel method and the influence of 1 h intermediate ball milling before calcination on different properties was investigated. The powders were studied by X-ray diffraction (XRD), scanning electron microscopy (SEM), differential thermal analysis (DTA), diffuse reflectance spectroscopy (DRS), photoluminescence (PL), and ultraviolet–visible spectrophotometer. Ball milling before calcination for 1 h was sufficient enough to accelerate the calcination process and obtain small size of the powder particles. Calcined ball milled sample at 450 °C for 2 h revealed anatase TiO2 phase structure with average particle size of 180 nm. The results showed that the synergistic effect of Cu doping and ball milling before calcination considerably narrowed the band gap of the synthesized TiO2 photocatalyst to 2.85 eV. PL results confirmed an effective separation of electron-hole pairs by Cu doping and also intermediated ball milling. The best photocatalytic performance under visible light in degradation of methylene blue (MB) was achieved with the combined influence of ball milling before calcination and Cu doping on TiO2, whereby a degradation of about 66% was obtained. It was found that the degradation reactions of MB by prepared photocatalysts followed the pseudo-first order reaction kinetics. In comparison, ball milling of TiO2 and Cu-doped TiO2 after calcination showed the undesirable effect on photocatalytic performance. In addition, nano-photocatalyst showed the good stability under visible light after recycling.

Cu-doped TiO2 nano-photocatalyst with an average particle size of 180 nm and a band gap of 2.85 eV was synthesized and MB degradation of about 66% was obtained after 120 min visible light irradiation.
Highlights
-
Cu-doped TiO2 powder was synthesized by combined sol–gel and mechanical milling.
-
Effect of 1 h intermediate ball milling before calcination was investigated.
-
Synergistic effect of Cu doping and ball milling narrowed the band gap of TiO2.
-
Effective separation of electron-hole pairs by Cu doping and milling was confirmed.
-
Photocatalytic degradation of 66% for methylene blue in visible light was obtained.












Similar content being viewed by others
References
Gupta SM, Tripathi M (2011) A review of TiO2 nanoparticles. Chinese Sci Bull 56:1639–1657
Yang XJ (2015) Preparation and photocatalytic performance of Cu-doped TiO2 nanoparticles. T Nonferr Metal Soc China 25:504–509
Carp O, Huisman CL, Reller A (2004) Photoinduced reactivity of titanium dioxide. Prog Solid State Chem 32:33–177
Wunderlich W (2004) Electronic properties of nano-porous TiO2 and ZnO thin films-comparision of simulations and experiments. J Ceram Process Res 5:343–354
Das D (2002) Preparation, physico-chemical characterization and catalytic activity of sulphated ZrO2-TiO2 mixed oxides. J Mol Catal A: Chem 189:271–282
Insebigler AL, Lu G, Yates Jr JT (1995) Photocatalysis on TiO2 surfaces: principles, mechanisms, and selected results. Chem Rev 95:735–758
Rampaul A (2003) Titania and tungsten doped titania thin films on glass; active photocatalysts. Polyhedron 22:35–44
Gracia F (2003) Optical and crystallisation behaviour of TiO2 and V/TiO2 thin films prepared by plasma and ion beam assisted methods. Thin Solid Films 429:84–90
Ghodsi F, Tepehan F, Tepehan G (2001) Study of time effect on the optical properties of spin-coated CeO2-TiO2 thin films. Sol Energy Mater Sol Cells 68:355–364
Almquist CB, Biswas P (2002) Role of synthesis method and particle size of nanostructured TiO2 on its photoactivity. J Catal 212:145–156
Sonawane R, Kale B, Dongare M (2004) Preparation and photo-catalytic activity of Fe/TiO2 thin films prepared by sol-gel dip coating. Mater Chem Phys 85:52–57
Oh J-H(2006) Effect of Ag nanoparticle size on the plasmonic photocatalytic properties of TiO2 thin films Surf Coat Technol 2:185–189
Nogawa T (2012) Preparation and visible-light photocatalytic activity of Au-and Cu-modified TiO2 powders. Mater Lett 82:174–177
Diwald O (2004) Photochemical activity of nitrogen-doped rutile TiO2 (110) in visible light. J Phys Chem B 108:6004–6008
Zhu J (2004) Characterization of Fe-TiO2 photocatalysts synthesized by hydrothermal method and their photocatalytic reactivity for photodegradation of XRG dye diluted in water. J Mol Catal A: Chem 216:35–43
Yu H, Irie H, Hashimoto K (2010) Conduction band energy level control of titanium dioxide: toward an efficient visible-light-sensitive photocatalyst. J Am Chem Soc 132:6898–6899
Wu NL, Lee MS (2004) Enhanced TiO2 photocatalysis by Cu in hydrogen production from aqueous methanol solution. Int J Hydrog Energy 29:1601–1605
Shen Y, Xiong T, Du H, Jin H, Shang J, Yang K (2009) Phosphorous, nitrogen, and molybdenum ternary co-doped TiO2: preparation and photocatalytic activities under visible light. J Sol-Gel Sci Technol 50:98–102
Facchin G, Carturan G, Campostrini R, Gialanella S, Lutterotti L, Armelao L, Marcì G, Palmisano L, Sclafani A (2000) Sol-gel synthesis and characterisation of TiO2-anatase powders containing nanometric platinum particles employed as catalysts for 4-nitrophenol photodegradation. J Sol-Gel Sci Technol 18:29–59
Hirakawa T, Kamat PV (2005) Charge separation and catalytic activity of Ag@ TiO2 core-shell composite clusters under UV-irradiation. J Am Chem Soc 127:3928–3934
Bingham S, Daoud WA (2011) Recent advances in making nano-sized TiO2 visible-light active through rare-earth metal doping. J Mater Chem 21:2041–2050
Arai T (2008) The enhancement of WO3-catalyzed photodegradation of organic substances utilizing the redox cycle of copper ions. Appl Catal B: Environ 84:42–47
Srinivas B (2011) Photocatalytic reduction of CO2 over Cu-TiO2/molecular sieve 5A composite. Photochem Photobiol 87:995–1001
Kuyumcu OK (2015) A comparative study for removal of different dyes over M/TiO2 (M=Cu, Ni, Co, Fe, Mn and Cr) photocatalysts under visible light irradiation. J Photochem Photobiol A: Chem 311:176–185
Lin WC, Yang WD (2012) Synthesis, Characterization and photocatalytic activity of copper (II)-doped titanium dioxide powders. Adv Mater Res 391-392:728–731
Kim DH, Hong HS, Kim SJ, Song JS, Lee KS (2004) Photocatalytic behaviors and structural characterization of nanocrystalline Fe-doped TiO2 synthesized by mechanical alloying. J Alloy Compd 375:259–264
Park HS (2006) The photocatalytic activity of 2.5 wt% Cu-doped TiO2 nano powders synthesized by mechanical alloying. J Alloy Compd 415:51–55
Cullity BD, Stock SR (2001) Elements of X-ray diffraction, 3rd edn. Prentice Hall, Upper Saddle River, NJ
Shafei A, Sheibani S (2019) Visible light photocatalytic activity of Cu doped TiO2-CNT nanocomposite powder prepared by sol-gel method. Mater Res Bull 110:198–206
Tauc J, Grigorovici R, Vancu A (1966) Optical properties and electronic structure of amorphous germanium. Phys Status Solidi 15:627–637
Chaurukaa SR, Hassanpour A (2015) Effect of mill type on the size reduction and phase transformation of gamma alumina. Chem Eng Sci 134:774–783
Handrick GR (1956) Heats of combustion of organic compounds. Ind Eng Chem 48:1366–1374
Eder D, Windle AH (2008) Morphology control of CNT-TiO2 hybrid materials and rutile nanotubes. J Mater Chem 18:2036–2043
Crişan D (2008) Crystallization study of sol-gel un-doped and Pd-doped TiO2 materials. J Phys Chem Solid 69:2548–2554
Yanagisawa K, Ovenstone J (1999) Crystallization of anatase from amorphous titania using the hydrothermal technique: effects of starting material and temperature. J Phys Chem B 103:7781–7787
Attar AS, Ghamsari MS (2008) Modifier ligands effects on the synthesized TiO2 nanocrystals. J Mater Sci 43:1723–1729
Zhou W, Zhou Y, Tang S (2005) Formation of TiO2 nano-fiber doped with Gd3+ and its photocatalytic activity. Mater Lett 59:3115–3118
Kim TW, Ha HW, Paek MJ, Hyun SH, Choy JH, Hwang SJ (2010) Unique phase transformation behavior and visible light photocatalytic activity of titanium oxide hybridized with copper oxide. J Mater Chem 20:3238–3245
Kubacka A, Munoz-Batista M, Fernández-García M, Obregón S, Colón G (2015) Evolution of H2 photoproduction with Cu content on CuOx-TiO2 composite catalysts prepared by a microemulsion method. Appl Catal B: Environ 163:214–222
Duhalde S, Vignolo MF, Golmar F, Chiliotte C, Torres CER, Errico LA, Cabrera AF, Rentería M, Sánchez FH, Weissmann M (2005) Appearance of room-temperature ferromagnetism in Cu-doped TiO2-δ films. Phys Rev B 72:161313–161318
Mathew S, Ganguly P, Rhatigan S, Kumaravel V, Byrne C, Hinder SJ, Bartlett J, Nolan M, Pillai SC (2018) Cu-doped TiO2: visible light assisted photocatalytic antimicrobial activity. Appl Sci 8:2067–2087
Kakhaki ZM, Youzbashi A, Naderi N (2015) Effect of milling energy and process ordering on the morphologies and optical properties of ZnO nanoparticles obtained through a mechanochemical technique. J Phys Sci 26:63–72
Liqiang J, Yichun Q, Baiqi W, Shudan L, Baojiang J, Libin Y, Wei F, Honggang F, Jiazhong S (2006) Review of photoluminescence performance of nano-sized semiconductor materials and its relationships with photocatalytic activity. Sol Energy Mater Sol Cells 90:1773–1787
Pugazhenthiran N, Murugesan S, Anandan A (2013) High surface area Ag-TiO2 nanotubes for solar/visible light photocatalytic degradation of ceftiofur sodium. J Hazard Mater 263:541–549
Kim T, Hur J, Jeon S (2016) The influence of interfacial defects on fast charge trapping in nanocrystalline oxide-semiconductor thin film transistors. Semicond Sci Technol 31:055014–055020
Raizada P, Singh P, Kumar A, Sharma G, Pare B, Jonnalagadda SB, Thakur P (2014) Solar photocatalytic activity of nano-ZnO supported on activated carbon or brick grain particles: role of adsorption in dye degradation. Appl Catal A: General 486:159–169
Zainal Z, Hui LK, Hussein MZ, Abdullah AH, Hamadneh IMKR (2009) Characterization of TiO2-chitosan/glass photocatalyst for removal of a monoazo dye via photodegradation-adsorption process. J Hazard Mater 164:138–145
Wang W, Chen X, Liu G, Shen Z, Xia D, Wong PK, Yu JC (2015) Monoclinic dibismuth tetraoxide: A new visible-light-driven photocatalyst for environmental remediation. Appl Catal B: Environ 176:444–453
Simsek EB (2017) Solvothermal synthesized boron doped TiO2 catalyst: photocatalytic degradation of endocrine disrupiting compounds and pharmaceuticals under visible light irradiation. Appl Catal B: Environmen 200:309–322
Acknowledgements
We would like to acknowledge the financial support of University of Tehran for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shafei, A., Salarpour, M.E. & Sheibani, S. Effect of intermediate ball milling on the synthesis of Cu-doped TiO2 nano-photocatalyst by sol–gel method. J Sol-Gel Sci Technol 92, 173–185 (2019). https://doi.org/10.1007/s10971-019-05045-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-019-05045-9