Abstract
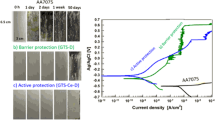
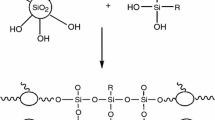
In this report, hybrid silica sol–gel coating was prepared using Ce(NO3)3 (Sol-Ce) as catalyst. Structure, surface morphology and anticorrosion ability of Sol-Ce solution/coating were studied by infrared spectroscopy (IR), scanning electron microscopy, energy dispersive spectrometer (EDS), potentiodynamic scan (PDS) and electrochemical impedance spectroscopy (EIS). IR results showed that Ce(NO3)3 had a stronger influence on reactivity of alkoxysilane. EDS results revealed that the fraction of Fe in Sol-Ce coating was significantly lower than regular acid-catalyzed hybrid silica sol–gel coating (Sol-Ac) and Sol-Ac with Ce(NO3)3-doped coating (Sol-Ac/Ce). The results of PDS and EIS demonstrated that Sol-Ce coating had better anticorrosion ability than Sol-Ac and Sol-Ac/Ce on carbon steel. The enhanced anticorrosion performance of the Sol-Ce coating might result from the following two reasons: (a) Ce(NO3)3 as a catalyst could alleviate the negative effects of low pH with using acid catalyst (dissolution of carbon steel) and (b) Ce(NO3)3 could impede corrosion of carbon steel as corrosion inhibitor.




Similar content being viewed by others
References
Wang D, Bierwagen GR (2009) Sol–gel coatings on metals for corrosion protection. Prog Org Coat 64(4):327–338. doi:10.1016/j.porgcoat.2008.08.010
Zheng SX, Li JH (2010) Inorganic-organic sol gel hybrid coatings for corrosion protection of metals. J Sol–Gel Sci Technol 54(2):174–187. doi:10.1007/s10971-010-2173-1
Zheludkevich ML, Salvado IM, Ferreira MGS (2005) Sol–gel coatings for corrosion protection of metals. J Mater Chem 15(48):5099–5111. doi:10.1039/B419153f
Metroke TL, Parkhill RL, Knobbe ET (2001) Passivation of metal alloys using sol–gel-derived materials—a review. Prog Org Coat 41(4):233–238. doi:10.1016/s0300-9440(01)00134-5
Duran A, Castro Y, Aparicio M, Conde A, de Damborenea JJ (2007) Protection and surface modification of metals with sol–gel coatings. Int Mater Rev 52(3):175–192. doi:10.1179/174328007x160263
Pathak SS, Khanna AS, Sinha TJM (2006) Sol–gel derived organic–inorganic hybrid coating: a new era in corrosion protection of material. Corros Rev 24(5–6):281–306. doi:10.1515/CORRREV.2006.24.5-6.281
Pepe A, Galliano P, Cere S, Aparicio M, Duran A (2005) Hybrid silica sol–gel coatings on Austempered Ductile Iron (ADI). Mater Lett 59(17):2219–2222. doi:10.1016/j.matlet.2005.03.001
Voevodin NN, Kurdziel JW, Mantz R (2006) Corrosion protection for aerospace aluminum alloys by Modified Self-assembled Nanophase Particle (MSNAP) sol–gel. Surf Coat Technol 201(3–4):1080–1084. doi:10.1016/j.surfcoat.2006.01.028
Campazzi E, Goletto V, Sanchez C (2007) Use of a nanostructured material, as protective coating of metal surfaces. WO 2007/119023 A2
Guo XH, An MZ (2010) Experimental study of electrochemical corrosion behaviour of bilayer on AZ31B Mg alloy. Corros Sci 52(12):4017–4027. doi:10.1016/j.corsci.2010.08.017
Schmidt H, Scholze H, Kaiser A (1984) Principles of hydrolysis and condensation reaction of alkoxysilanes. J Non-Cryst Solids 63(1–2):1–11. doi:10.1016/0022-3093(84)90381-8
Pu ZC, vanOoij WJ, Mark JE (1997) Hydrolysis kinetics and stability of bis(triethoxysilyl)ethane in water–ethanol solution by FTIR spectroscopy. J Adhes Sci Technol 11(1):29–47. doi:10.1163/156856197X01001
Wen JY, Wilkes GL (1996) Organic/inorganic hybrid network materials by the sol–gel approach. Chem Mater 8(8):1667–1681. doi:10.1021/cm9601143
Van Ooij W, Zhu D, Stacy M, Seth A, Mugada T, Gandhi J, Puomi P (2005) Corrosion protection properties of organofunctional silanes—an overview. Tsinghua Sci Technol 10(6):639–664. doi:10.1016/S1007-0214(05)70134-6
Osterholtz FD, Pohl ER (1992) Kinetics of the hydrolysis and condensation of organofunctional alkoxysilanes: a review. J Adhes Sci Technol 6(1):127–149. doi:10.1163/156856192X00106
Arkles B, Steinmetz JR, Zazyczny J, Mehta P (1992) Factors contributing to the stability of alkoxysilanes in aqueous-solution. J Adhes Sci Technol 6(1):193–206. doi:10.1163/156856192X00133
Brinker CJ, Scherer GW (1990) Sol–gel science: the physics and chemistry of sol–gel processing. Academic Press, New York
Correa PS, Malfatti CF, Azambuja DS (2011) Corrosion behavior study of AZ91 magnesium alloy coated with methyltriethoxysilane doped with cerium ions. Prog Org Coat 72(4):739–747. doi:10.1016/j.porgcoat.2011.08.005
Aramaki K (2001) Treatment of zinc surface with cerium(III) nitrate to prevent zinc corrosion in aerated 0.5 M NaCl. Corros Sci 43(11):2201–2215. doi:10.1016/S0010-938X(00)00189-X
Onofre-Bustamante E, Dominguez-Crespo MA, Torres-Huerta AM, Olvera-Martinez A, Genesca-Llongueras J, Rodriguez-Gomez FJ (2009) Characterization of cerium-based conversion coatings for corrosion protection of AISI-1010 commercial carbon steel. J Solid State Electrochem 13(11):1785–1799. doi:10.1007/s10008-009-0871-9
Fahrenholtz WG, O’Keefe MJ, Zhou HF, Grant JT (2002) Characterization of cerium-based conversion coatings for corrosion protection of aluminum alloys. Surf Coat Technol 155(2–3):208–213. doi:10.1016/s0257-8972(02)00062-2
Pepe A, Aparicio M, Cere S, Duran A (2004) Preparation and characterization of cerium doped silica sol–gel coatings on glass and aluminum substrates. J Non-Cryst Solids 348:162–171. doi:10.1016/j.jnoncrysol.2004.08.141
Cere S, Pepe A, Aparicio M, Duran A (2006) Cerium hybrid silica coatings on stainless steel AISI 304 substrate. J Sol–Gel Sci Technol 39(2):131–138. doi:10.1007/s10971-006-9173-1
Wang H, Akid R (2008) Encapsulated cerium nitrate inhibitors to provide high-performance anti-corrosion sol–gel coatings on mild steel. Corros Sci 50(4):1142–1148. doi:10.1016/j.corsci.2007.11.019
Rubio F, Rubio J, Oteo JL (1998) A FT-IR study of the hydrolysis of tetraethylorthosilicate (TEOS). Spectrosc Lett 31(1):199–219. doi:10.1080/00387019808006772
Innocenzi P (2003) Infrared spectroscopy of sol–gel derived silica-based films: a spectra-microstructure overview. J Non-Cryst Solids 316(2–3):309–319. doi:10.1016/S0022-3093(02)01637-X
Zucchi F, Grassi V, Frignani A, Trabanelli G, Monticelli C (2007) Octadecyl-trimethoxy-silane film formed on copper in different conditions. Mater Chem Phys 103(2–3):340–344. doi:10.1016/j.matchemphys.2007.02.050
Brinker CJ (1988) Hydrolysis and Condensation of Silicates—Effects on Structure. J Non-Cryst Solids 100(1–3):31–50. doi:10.1016/0022-3093(88)90005-1
Goldstein J, Newbury DE, Joy DC, Lyman CE, Echlin P, Lifshin E, Sawyer L, Michael JR (2003) Scanning electron microscopy and X-ray microanalysis, 3rd edn. Springer, New York
Song J, Van Ooij WJ (2003) Bonding and corrosion protection mechanisms of gamma-APS and BTSE silane films on aluminum substrates. J Adhes Sci Technol 17(16):2191–2221. doi:10.1163/156856103772150788
Bard AJ, Faulkner LR (2001) Electrochemical methods: fundamentals and applications, 2nd edn. Wiley, New Jersey
Peng S, Zeng Z, Zhao W, Li H, Xue Q, Wu X (2012) Synergistic effect of thiourea in epoxy functionalized silica sol–gel coating for copper protection. Surf Coat Technol 213:175–192. doi:10.1016/j.surfcoat.2012.10.043
Acknowledgments
This work was supported by the NSFC (51105356), the Zhejiang Provincial Natural Science Foundation (Y4100488), the Ningbo Natural Science Foundation (2011A610160) and the “Outstanding Talent Recruiting Program” (2009A31004) dedicated to Academician Qunji Xue from Ningbo Municipal Government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peng, S., Zhao, W., Zeng, Z. et al. Preparation of anticorrosion hybrid silica sol–gel coating using Ce(NO3)3 as catalyst. J Sol-Gel Sci Technol 66, 133–138 (2013). https://doi.org/10.1007/s10971-013-2976-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-013-2976-y