Abstract
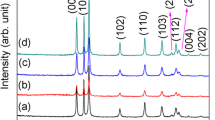
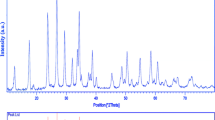
Various morphologies of ZnO nanostructures, such as nanoparticles, nanorods and nanoflowers have been achieved controllably by polymeric sol–gel method. In this approach, zinc nitrate Zn(NO3)2·6H2O, citric acid and ethylene glycol were used as the source of Zn2+, the chelating agent and the solvent agent, respectively. The microstructure of the ZnO nanostructures was characterized by X-ray diffractometry, scanning electron microscopy with the energy dispersive X-ray spectroscopy, transmission electron microscopy, thermogravimetric analysis and Fourier transform infrared spectroscopy. The effect of ethylene glycol to citric acid mole ratio on the morphology and structure of the products was discussed. The ZnO nanoparticles with diameter between 24 ± 2 nm was obtained with EG:CA mole ratio equal to 2:1. The optical properties of as-obtained power were investigated by ultraviolet–visible spectroscopy.








Similar content being viewed by others
References
Xia Y, Yang P, Sun Y, Wu Y, Mare B, Gates B, Yin Y, Kim F, Yan H (2003) Adv Mater 15:323–327
Salavati-Niasari M, Davar F, Loghman-Estarki MR (2009) J Alloys Compd 475:782–788
Bouropoulos N, Tsiaoussis I, Poulopoulos P, Roditis P, Baskoutas S (2008) Mater Lett 62:3533–3535
Salavati-Niasari M, Loghman-Estarki MR, Davar F (2008) Chem Eng J 145:346–350
Gao PX, Wang ZL (2003) J Am Chem Soc 125(11299):11305
Baruah S, Dutta JD (2009) Sci Technol Adv Mater 10:013001–0130019
Wang ZL (2004) Mater Today 7(26):33
He G, Cai JH, Ni G (2008) Mater Chem Phys 110(110):114
Salavati-Niasari M, Davar F, Farhadi M (2009) J Sol-Gel Sci Technol 51(48):52
Pechini MP (1967) Patent US no. 3 330 697
Sakka S, Kozuka H (2005) Handbook of sol–gel science and technology processing, characterization and applications. Kluwer, Dordrecht, pp 60–76
Salavati-Niasari M, Davar F, Loghman-Estarki MR (2010) J Alloys Compd 494(199):204
Wu L, Wu Y, Lü W (2005) Phys E 28(76):82
Zhang YC, Wu X, Hu XY, Guo R (2005) J Cryst Growth 280(250):254
Ristić M, Musić S, Ivanda M, Popovíć S (2005) J Alloys Compd 397:L1–L4
Li J, Srinivasan S, He GN, Kang JY, Wu ST, Ponce FA (2008) J Cryst Growth 310(599):603
Lee J, Easteal AJ, Pal U, Bhattacharyya D (2009) Curr Appl Phys 9(792):796
Shoja Razavi R, Loghman Estarki MR et al (2010) Curr Nano Sci 7:807–812
Rani S, Suri P, Shishodia PK, Mehra RM (2008) Sol Energy Mat Sol Cells 92(1639):1645
Chezhina NV, Korolev DA (2011) Open Fuel Cells J 4(7):15
Shi M, Xu Y, Liu A, Liu N, Wang C, Majewski P, Aldinger F (2009) Mater Chem Phys 114(43):46
Liang S, Sheng H, Liu Y, Hio Z, Lu Y, Chen H (2001) J Cryst Growth 225:110
Saito N, Haneda H, Sekiguchi T, Ohashi N, Sakaguchi I, Koumoto K (2002) Adv Mater 14:418
Sánchez C, Doria J, Paucar C, Hernandez M, Squera AM, Rodríguez JE, Gómez A, Baca E, Morán O (2010) Phys B 405:3679–3684
Singh KA, Pathak LC, Roy SK (2007) Ceram Inter 33(1463):1468
Mondelaers D, Vanhoyland G, Van den Rul H, Haen JD, Van Bael MK, Mullens J, Van Poucke LC (2002) Mater Res Bull 37(901):914
Acknowledgments
The authors would like to acknowledge Malek Ashtar University of Technology, department of material engineering, for the financial support. One of the authors would like thanks Dr. F. Davar for her excellent comments for improving quality of this manuscript. In final, the corresponding author would like to present this work to his daughter Sara.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farhadi-Khouzani, M., Fereshteh, Z., Loghman-Estarki, M.R. et al. Different morphologies of ZnO nanostructures via polymeric complex sol–gel method: synthesis and characterization. J Sol-Gel Sci Technol 64, 193–199 (2012). https://doi.org/10.1007/s10971-012-2847-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-012-2847-y