Abstract
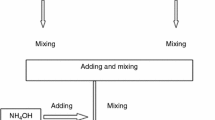
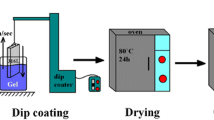
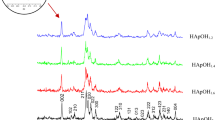
Hydroxyapatite Ca10(PO4)6(OH)2 has attracted widespread interest from both orthopedic and dental fields due to its excellent biocompatibility and tissue bioactivity properties. Since nanophase materials can mimic the dimensions of constituent components of natural tissues, the implants developed from nanophase material could serve as a successful alternative. However, the defects of hydroxyapatite ceramics, mainly brittleness and low fracture toughness, have been overcome by the use of nanophase hydroxyapatite coatings on the implant surfaces that integrate the good mechanical properties of metals and the bioactivity of hydroxyapatite. In the present investigation, Sol–gel hydroxyapatite was prepared from two different phosphorus precursors such as triethyl phosphate and phosphorus pentoxide respectively with calcium nitrate tetrahydrate as a calcium precursor. The effects of pH and liquid P31 Nuclear Magnetic Resonance spectroscopy for the solution aged at different periods were investigated and the synthesized hydroxyapatite powder was characterized by Transmission electron microscopy, X-ray Powder Diffraction, Fourier transform infrared spectroscopy and thermal analysis respectively. In order to fully understand the bioactivity of the synthesized materials, they were coated on 316L Stainless Steel implant surface by spin coating method at the spin speed of 2,000 Revolutions per minute. The effect of nanoparticles on the surface of 316L Stainless Steel implant was studied by adhesive strength measurements. The corrosion resistance property of the hydroxyapatite coatings was evaluated by electrochemical impedance analysis. From the results, it was observed that the hydroxyapatite coatings obtained from different precursors have very high resistance to corrosion with higher adhesive strength.








Similar content being viewed by others
References
Rho JY, Kuhn-Spearing L, Zioupos P (1998) Med Eng Phys 20:92
Clemens JA, Klein CP, Vriesde RC, Rozing PM, de Groot K (1998) J Biomed Mater Res 40:341
Bezzi G, Celotti G, Landi E, La Torretta TMG, Sopyan I, Tampieri A (2003) Mater Chem Phys 78:816
Mavis B, Tas AC (2000) J Am Ceram Soc 83:989
Huang YY, Chou KS (2003) Ceram Int 29:485
Paul W, Sharma CP (2006) Am J Biochem Biotechnol 2:41
Pang YX, Bao X (2003) J Eur Ceram Soc 23:1697
Kim HW, Koh YH, Li LH, Lee S, Kim HE (2004) Biomaterials 25:2533
Kim HW, Kong YM, Bae CJ, Noh YJ, Kim HE (2004) Biomaterials 25:2919
Seok Kim II, Kumta PN (2004) Mater Sci Eng B 111:232
Weng W, Baptista JL (1999) J Am Ceram Soc 82:27
Tian F, Pan L, Wu X, Wu F (1988) J Non Cryst Solids 104:129
Gross KA, Hanley L, Chai CS, Kannangara K, Ben-Nissan B (1998) J Mater Sci Mater Med 9:839
Westheimer FH, Huang S, Coritz F (1988) J Am Chem Soc 110:181
Young RS, Holcomb DW (1982) Calif Tissue Int 34:17
Livage J, Barboux P, Vandenborre MT, Schmutz C, Taulell F (1992) J Non Cryst Solids 147/148:18
Anee TK, Ashok M, Palnichamy M, Kalkura SN (2003) Mater Chem Phys 80:725
Liu DM, Yang Q, Troczynski T, Tseng WJ (2002) Biomaterials 23:1679
Stoch A, Jastrzebski W, Brozek A, Stoch J, Szaraniec J, Trybalska B, Kmita G (2000) J Mol Struct 555:375
Debruijn JD, Bovell YP, Van blitterswijk CA (1994) Biomaterials 15:543
Blakeslee KC, Condrate RA (1971) J Am Ceram Soc 54:559
Yanbao Li, Li Dongxu (2008) Int J Appl Ceram Technol 5:442
Rieu J (1993) Clin Mater 12:227
De Groot K, Klein CPAT, Wolke JGC, de Bliek-Hogervost JMA (1990) Chemistry of calcium phosphate bioceramics. In: Yamamuro T, Hench LL, Wilson J (eds) Handbook of bioactive ceramics, vol II. CRC Press, Boca Raton, Florida
Tas AC (2000) J Eur Ceram Soc 20:2389
Park JB, Lakes RS (1992) Biomaterials: an introduction. Plenum Press, New York
Yang YC, Chang E, Lee SY (2003) J Biomed Mater Res Part A 67:886
Vijayalakshmi U, Rajeswari S (2007) J Solgel Sci Technol 43:251
Vijayalakshmi U, Prabakaran K, Rajeswari S (2008) J Biomed Mater Res Part A 87(A):739
Uhlmann DR, Suratwala T, Davidson K, Boulton JM, Teowee G (1997) J Non Cryst Solids 218:113
Thangaraj V, Eliaz N, Chitharanjan Hegde A (2009) J Appl Electrochem 39:339
Catledge SA, Fries MD, Vohra atledge SA, Fries MD, Vohra YK, Lace field WR, Lemons KE, Woodard S, Venugopalan R (2002) J Nano Sci Nano Technol 2:293
Amato LE, Lopez DA, Galliano PG, Cere SM (2005) Mater Lett 59:2026
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vijayalakshmi, U., Rajeswari, S. Influence of process parameters on the sol–gel synthesis of nano hydroxyapatite using various phosphorus precursors. J Sol-Gel Sci Technol 63, 45–55 (2012). https://doi.org/10.1007/s10971-012-2762-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-012-2762-2