Abstract
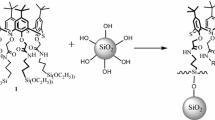
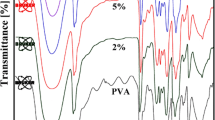
Different surface modification methods to improve the dispersity of nano-silica in organic solvents by γ-methacryloxypropyltrimethoxy silane (MOPTMS) anhydrous alcohol solution were investigated. The possible principles of these methods improving the dispersity of nano-silica in organic solvents were proposed. The optimum modification method was that nano-silica powders were chemically modified by γ-methacryloxypropyltrimethoxy silane anhydrous alcohol solution and γ-methacryloxypropyltrimethoxy silane anhydrous alcohol solution was dropwise added at 3 mL/min. The transparency of the optimum modified nano-silica powder anhydrous alcohol solution (0.1 wt. %) was 85.9%. The 29Si cross polarization and magic-angle spinning nuclear magnetic resonance spectra suggested that nano-silica was chemically modified by γ-methacryloxypropyltrimethoxy silane anhydrous alcohol solution. The transmission electron microscopy images suggested that the optimum modified nano-silica was well dispersed. The dispersity of nano-silica in anhydrous alcohol, CH2Cl2, CCl4, cyclohexane, and liquid paraffin increased from 0, 0, 0, 0, and 0.1 to 9.4, 9.6, 9.8, 9.8, and 10.0 g/100 mL after surface modification.



Similar content being viewed by others
References
Gao Guimei, Zhou Haifeng, Gan Shucai (2008) Pow Tech 7:190–197
Bhagat SharadD, Venkateswara Rao A (2006) App Sur Sci 252:4289–4297
Shi Fei, Wang Lijiu, Liu Jingxiao (2006) Mater Lett 60:3718–3722
Zhao Li, Jiaguo Yu, Cheng Bei (2005) J Non Crys Solid 351:3593–3599
Zhao Li, Yu Jiaguo (2006) J Coll Inter Sci 304:84–91
Yulin Li, Chen Zhengxing, Li Xuanxiao (2011) Asi J Chem 23:893–898
Voelkel A (2000) Chroma 51:608–614
Zongwei Li, Zhu Yongfa (2003) App Sur Sci 211:315–320
Su Henglei (2007) J App Poly Sci 103:3600–3608
Takai Chika (2007) Col Sur A Phys Eng Asp 292:79–82
Xiaohong Li, Cao Zhi, Zhang Zhijun (2006) App Sur Sci 252:7856–7861
Kalapathy U, Proctor A, Shultz J (2002) Biore Tech 85:285–289
Tang Qi, Wang Tao (2005) J Super Flu 35:91–94
Ting Li, Wang Tao (2008) Mater Chem Phy 5:252–256
Zou Hua, Shishan Wu, Shen Jian (2008) Chem Rev 108:3942–3945
Kalapathy U, Proctor A, Shultz J (2000) Biore Tech 73:257–262
Zou Jianhua, Shi Wenfang, Hong Xiaoyin (2005) Comp. Part A Appl Sci Manufac 36:631–637
Jiaguo Yu, Guo Hongtao, Davis SeanA (2006) Adv Funct Mater 16:2035–2041
Acknowledgments
This study was financially supported by Earmarked Fund for Modern Agro-industry Technology Research System, Innovation Project of Education Ministry (No. IRT0627), and Food Processing Technology Research (No. 2006BAD05A10).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, Y., Chen, Z., Li, X. et al. A new surface modification method to improve the dispersity of nano-silica in organic solvents. J Sol-Gel Sci Technol 58, 290–295 (2011). https://doi.org/10.1007/s10971-010-2389-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-010-2389-0