Abstract
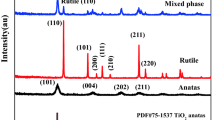
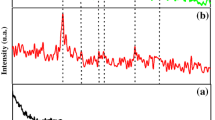
In order to clarify the effects of diethanolamine (DEA) in the silver (Ag)/titanium dioxide (TiO2) sol–gel process, sols with and without DEA, and films derived from these sols were prepared. The samples were investigated by X-ray diffraction, transmission electron microscopy, electron diffraction and optical absorption spectra. The results showed that metallic Ag clusters were formed in the sol with DEA and was absent in the sol without DEA. This indicated that DEA worked not only as the stabilizer but also as the reduce agent in Ag/TiO2 sol–gel process. After annealed, Ag metallic nanoparticles were generated in the films derived from both the sols with and without DEA. The particles in the films derived from the sol with DEA were smaller than those from the sol without DEA. This can be ascribed to the limitation of the growth of Ag cluster formed in the sol with DEA during heat treatment. Mechanisms for the formation of metallic Ag in the Ag/TiO2 sols and films were discussed. The effects of DEA in the sols and films were studied in detail.









Similar content being viewed by others
Abbreviations
- DEA or deaH2 :
-
Diethanolamine
- TEA:
-
Triethanolamine
References
Oregan B, Gratzel M (1991) A low-cost, high-efficiency solar-cell based on dye-sensitized colloidal TiO2 films. Nature 353:737–740
Arena A, Donato N, Saitta G, Rizzo G, Neri G, Pioggia G (2007) Photosensitive heterojunctions of silicon coated with sol-gel derived TiO2 dispersed in poly(3, 4-ethylendi oxythiophene)/poly(styrenesulfonate). J Sol-Gel Sci Technol 43:41–46
Li H, Zhao GL, Han GR, Song B (2007) Hydrophilicity and photocatalysis of Ti1-xVxO2 films prepared by sol-gel method. Surf Coat Technol 201:7615–7618
Hocevar M, Berginc M, Topic M, Krasovec UO (2010) Sponge-like TiO2 layers for dye-sensitized solar cells. J Sol-Gel Sci Technol 53:647–654
Deng LX, Chen YL, Yao MY, Wang SR, Zhu BL, Huang WP, Zhang SM (2010) Synthesis, characterization of B-doped TiO2 nanotubes with high photocatalytic activity. J Sol-Gel Sci Technol 53:535–541
Cao BS, Feng ZQ, He YY, Li H, Dong B (2010) Opposite effect of Li + codoping on the upconversion emissions of Er3 + -doped TiO2 powders. J Sol-Gel Sci Technol 54:101–104
Bottcher H, Mahltig B, Sarsour J, Stegmaier T (2010) Qualitative investigations of the photocatalytic dye destruction by TiO2-coated polyester fabrics. J Sol-Gel Sci Technol 55:177–185
Bell AT (2003) The impact of nanoscience on heterogeneous catalysis. Science 299:1688–1691
Carp O, Huisman CL, Reller A (2004) Photoinduced reactivity of titanium dioxide. Prog Solid State Ch 32:33–177
Sopyan I, Watanabe M, Murasawa S, Hashimoto K, Fujishima A (1996) An efficient TiO2 thin-film photocatalyst: photocatalytic properties in gas-phase acetaldehyde degradation. J Photochem Photobiol A 98:79–86
Xin BF, Ren ZY, Hu HY, Zhang XY, Dong CL, Shi KY, Jing LQ, Fu HG (2005) Photocatalytic activity and interfacial carrier transfer of Ag-TiO2 nanoparticle films. Appl Surf Sci 252:2050–2055
Zhao GL, Kozuka H, Yoko T (1996) Sol-gel preparation and photoelectrochemical properties of TiO2 films containing Au and Ag metal particles. Thin Solid Films 277:147–154
Zhao GL, Kozuka H, Yoko T (1997) Effects of the incorporation of silver and gold nanoparticles on the photoanodic properties of rose Bengal sensitized TiO2 film electrodes prepared by sol-gel method. Sol Energ Mat Sol C 46:219–231
Sarkar J, John VT, He J, Brooks C, Gandhi D, Nunes A, Ramanath G, Bose A (2008) Surfactant-templated synthesis and catalytic properties of patterned nanoporous Titania supports loaded with platinum nanoparticles. Chem Mater 20:5301–5306
Rodriguez JA, Evans J, Graciani J, Park JB, Liu P, Hrbek J, Sanz JF (2009) High water-gas shift activity in TiO2(110) supported Cu and Au nanoparticles: role of the oxide and metal particle size. J Phys Chem C 113:7364–7370
Li H, Zhao GL, Chen ZJ, Song B, Han GR (2010) TiO2-Ag nanocomposites by low-temperature sol-gel processing. J Am Ceram Soc 93:445–449
Roy R (1987) Citation-classic—aids in hydrothermal experimentation.2. Methods of making mixtures for both dry and wet phase-equilibrium studies. Cc/Eng Tech Appl Sci 33:16
Lu X, Imae T (2007) Size-controlled in situ synthesis of metal nanoparticles on dendrimer-modified carbon nanotubes. J Phys Chem C 111:2416–2420
Zhang YW, Peng HS, Huang W, Zhou YF, Zhang XH, Yan DY (2008) Hyperbranched poly(amidoamine) as the stabilizer and reductant to prepare colloid silver nanoparticles in situ and their antibacterial activity. J Phys Chem C 112:2330–2336
Tian CG, Mao BD, Wang EB, Kang ZH, Song YL, Wang CL, Li SH (2007) Simple strategy for preparation of core colloids modified with metal nanoparticles. J Phys Chem C 111:3651–3657
Traversa E, Di Vona ML, Nunziante P, Licoccia S, Yoon JW, Sasaki T, Koshizaki N (2001) Photoelectrochemical properties of sol-gel processed Ag-TiO2 nanocomposite thin films. J Sol-Gel Sci Technol 22:115–123
Verma A, Kar M, Agnihotry SA (2007) Aging effect of diethanolamine stabilized sol on different properties of TiO2 films: Electrochromic applications. Sol Energ Mat Sol C 91:1305–1312
Huang MH, Choudrey A, Yang PD (2000) Ag nanowire formation within mesoporous silica. Chem Commun 12:1063–1064
Singh A, Mehrotra RC (2004) Novel heterometallic alkoxide coordination systems of polyols (glycols, di- and tri-ethanolamines) derived from the corresponding homometallic moieties. Coordin Chem Rev 248:101–118
Kocareva T, Grozdanov I, Pejova B (2001) Ag and AgO thin film formation in Ag+-triethanolamine solutions. Mater Lett 47:319–323
Wang SZ, Xin HW (2000) Fractal and dendritic growth of metallic Ag aggregated from different kinds of gamma-irradiated solutions. J Phys Chem B 104:5681–5685
Acknowledgments
This work is supported by Zhejiang Research Department, under grant No. Y200909120.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, J., Zhao, G., Ren, X. et al. Effects of diethanolamine on the evolution of silver/titanium dioxide sol–gel process. J Sol-Gel Sci Technol 58, 148–155 (2011). https://doi.org/10.1007/s10971-010-2369-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-010-2369-4