Abstract
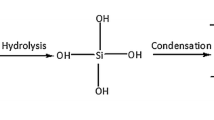
Hybrid melting gels were prepared by a sol–gel process, starting with a mono-substituted siloxane and a di-substituted siloxane. Methyl-modified melting gels were prepared using (a) methyltriethoxysilane (MTES) with dimethyldiethoxysilane (DMDES) and (b) methyltrimethoxysilane (MTMS) together with dimethyldimethoxysilane (DMDMS). The gels with MTES–DMDES were prepared with concentrations between 50–50 and 75–25 mol%. The gels with MTMS–DMDMS were prepared with concentrations between 50–50 and 70–30 mol%. For both systems, the consolidation temperature, after which the melting gel no longer softens, increased with an increase in the amount of the mono-substituted siloxane, increasing from 135 to 160 °C for MTES–DMDES and increasing from 145 to 170 °C for MTMS–DMDMS. Coatings formed on mica substrates were about 1 mm thick, and showed no visible cracks. The surfaces of the coatings were profiled using micro-Raman spectroscopy, which revealed that methyl groups were concentrated at the surfaces of the films. All contact angles measured with water were greater than 90°.





Similar content being viewed by others
References
Gunji T, Iizuka Y, Arimitsu K, Abe Y (2004) Preparation and properties of alkoxy(methyl)silsesquioxanes as coating agents. J Polym Sci A Polym Chem 42:3676–3684
Ghisleni R, Lucca DA, Wang YQ, Lee J-K, Nastasi M, Dong J, Maher A (2008) Ion irradiation effects on surface mechanical behavior and shrinkage of hybrid sol–gel derived silicate thin films. Nucl Instr Meth Phys Res B 266:2433–2456
Dong H, Brook MA, Brennan JD (2005) A new route to monolithic methylsilsesquioxanes: gelation behavior of methyltrimethoxysilane and morphology of resulting methylsilsesquioxanes under one-step or two-step processing. Chem Mater 17:2807–2816
Dong H, Brennan JD (2006) Macroporous monolithic methylsilsesquuioxanes prepared by a two-step acid/acid processing method. Chem Mater 18:4176–4182
Katayama S, Nonaka Y, Iwata K, Kubo Y, Yamada N (2005) Synergetic effect of inorganic and organic components on solid acid/base properties of organosiloxane-based inorganic hybrid materials. Adv Mater 17:2596–2599
Xu Y, Liu R, Wu D, Sun Y, Gao H, Yuan H, Deng F (2005) Ammonia-catalyzed hydrolysis kinetics of mixture of tetraethoxysilane with methyltriethoxysilane by 29Si NMR. J Non-Cryst Solids 351:2403–2413
Orel B, Jese R, Vilcnik A, Lavrencic-Stangar U (2005) Hydrolysis and solvolysis of methyltriethoxysilane catalyzed with HCl or trifluoroacetic acid: IR spectroscopic and surface energy studies. J Sol–Gel Sci Tech 34:251–265
Liu R, Xu Y, Wu D, Sun Y, Gao H, Yuan H, Deng F (2004) Comparative study on hydrolysis kinetics of substituted ethoxysilanes by liquid-state 29Si-NMR. J Non-Cryst Solids 343:61–70
Hass KH, Amberg-Schwab S, Rose K (1999) Functionalization coating materials based on inorganic–organic polymers. Thin Solid Films 351:198–203
Jeong S, Ahn S-J, Moon J (2005) Fabrication of patterned inorganic–organic hybrid film for the optical waveguide by microfluidic lithography. J Am Ceram Soc 88:1003–1036
Matsuda A, Matsuno Y, Tatsumisago M, Minami T (1998) Fine pattering and characterization of gel films derived from methyltriethoxysilane and tetraethoxysilane. J Am Ceram Soc 81:2849–2852
Kwok DY, Neumann AW (1999) Contact angle measurements and contact angle interpretation. Adv Colloid Interface Sci 8:167–249
Chen W, Fadeev AY, Hsieh MC, Öner D, Youngblood J, McCarthy TJ (1999) Ultrahydrophobic and ultralyophobic surfaces: some comments and examples. Langmuir 15:3395–3399
Castricum HL, Sah A, Mittelmeijer-Hazeleger MC, Huiskes C, ten Elshof JE (2007) Microstructure and enhanced hydrophobicity in methylated SiO2 for molecular separation. J Mater Chem 17:1509–1517
Shirtclieffe NJ, McHale G, Newton MI, Perry CC, Roach P (2005) Porous materials show superhydrophobic to superhydrophilic switching. Chem Commun 3135–3137
Matsuda A, Sasaki T, Hasegawa K, Tatsumisago M, Minami T (2001) Thermal softening behavior and application to transparent thick films of poly(benzylsilsesquioxane) particles prepared by sol–gel process. J Am Ceram Soc 84(4):775–780
Masai H, Tokuda Y, Yoko T (2005) Gel-melting method for preparation of organically modified siloxane low-melting glasses. J Mater Res 20(5):1234–1241
Kakiuchida H, Takahashi M, Tokuda Y, Masai H, Kuniyoshi M, Yoko T (2006) Viscoelastic and structural properties of the phenyl-modified polysiloxane system with a three-dimensional structure. J Phys Chem B 110:7321–7327
Kakiuchida H, Takahashi M, Tokuda Y, Masai H, Kuniyoshi M, Yoko T (2007) Effects of organic groups on structure and viscoelastic properties of organic–inorganic polysiloxane. J Phys Chem B 111:982–988
Jitianu A, Doyle J, Amatucci G, Klein LC (2008) Methyl-modified melting gels for hermetic barrier coatings. Proceedings MS&T 2008 enabling surface coating systems: multifunctional coatings (CD-ROM), Pittsburgh, PA, pp 2171–2182
Jitianu A, Britchi A, Badescu V, Deleanu C, Zaharescu M (2007) Influence of the alkoxy group of the Si-alkoxides on the sol–gel process and on the structure of the obtained gels. Rev Roum Chim 52(1–2):93–99
Chomel AD, Dempsey P, Latournerie J, Hourlie-Bahloul D, Jayasooriya UA (2005) Gel to glass transformation of methytriethoxysilane: a silicone oxycarbide glass precursor investigated using vibrational spectroscopy. Chem Mate 17:4468–4473
Das G, Bettotti P, Ferraioli L, Raj R, Mariotto G, Pavesti L, Soraru GD (2007) Study of pyrolysis process of an hybrid CH3SiO1.5 gel into a SiCO glass. Vib Spectrosc 45:61–68
Bertoluzza A, Fagnano C, Morelli MA, Gottardi V, Guglielmi M (1982) Raman and infrared spectra on silica gel evolving towards glass. J Non-Cryst Solids 48:117–128
Capozzi CA, Pye LD, Condrate RA Sr (1992) Vibrational spectral/structural changes from the hydrolysis/polycondensation of methyl-modified silicates I. Comparisons for single monomer condensates. Mat Lett 15:130–136
Pakjamsai C, Kobayashi N, Koyano M, Sasaki S, Kawakami Y (2004) Characterization of the benzene-insoluble fraction of the hydrolyzate of phenyltrimethoxysilane in the presence of benzyltrimethylamonium hydroxide. J Polym Sci A Polym Chem 42:4587–4597
Riegel B, Plittersdorf S, Kiefer W, Hüsing N, Schubert U (1997) Raman spectroscopic analysis of the sol–gel processing of RSi(OMe)3/Si(OMe)4 mixtures. J Mol Struct 410–411:157–160
Takahashi K, Tadanaga K, Hayashi A, Matsuda A, Tatsumisago M (2007) Effect of phenyltriethoxysilane concentration in starting solutions on thermal properties of polyphenylsilsesquioxane particles prepared by a two-step acid–base catalyzed sol–gel process. J Ceram Soc Jpn 115(2):131–135
Takahashi K, Tadanaga K, Matsuda A, Hayashi A, Tatsumisago M (2007) Thermoplastic and thermosetting properties of polyphenylsilsesquioxane particles prepared by two step acid–base catalyzed sol–gel process. J Sol–Gel Sci Tech 41:217–222
Jitianu A, Amatucci G, Klein LC (2008) Phenyl-substituted siloxane hybrid gels that soften below 140 °C. J Am Ceram Soc 92:36–40
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jitianu, A., Doyle, J., Amatucci, G. et al. Methyl modified siloxane melting gels for hydrophobic films. J Sol-Gel Sci Technol 53, 272–279 (2010). https://doi.org/10.1007/s10971-009-2087-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-009-2087-y