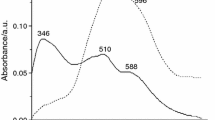
Abstract
A watersoluble citratoperoxo–Ti(IV) complex was synthesized and the chemical reactions that occur during the different synthesis steps were studied. A crystalline complex could be obtained by slow evaporation of highly concentrated precursor solutions, while fast evaporation led to the preparation of a homogeneous, amorphous glassy solid, the gel. The chemical structure of the crystallized complex ion and of the gel were shown to be related, but differed due to the presence of a crosslinked ammonium citrate network in the gel, which prevents crystallization. The thermo-oxidative decomposition pathway of the crystalline complex and the gel to the metal oxide is described based on in-situ thermal analysis techniques combined with Fourier transform infrared and mass spectrometry, diffuse reflection infrared by Fourier transform as well as high-temperature X-ray diffraction. The study offers a fundamental understanding of the behavior of the aqueous solution gel Ti(IV) precursor which is applicable in the preparation of all kinds of multimetal oxide systems containing Ti(IV) ions.












Similar content being viewed by others
References
Schwartz RW, Schneller T, Waser R (2004) C R Chim 7:433
Kakihana M, Szanics J, Tada M (1999) Bull Korean Chem Soc 20:893
Narendar Y, Messing GL (1997) Chem Mater 9:580
Van den Rul H, Mondelaers D, Van Bael MK, Mullens J 2006 J Sol-Gel Sci Technol 39:41
Nelis D, Calderon-Moreno JM, Popa M, van Bael MK, Mullens J, van Poucke LC (2006) J European Ceram Soc 26:409
Hardy A, Vanhoyland G, Van Genechten D, Van Bael M, Van den Rul H, Mullens J, D’Haen J, Goux L, Wouters D (2007) J Sol-Gel Sci Techn 42:239
Van Werde K, Vanhoyland G, Mondelaers D, Den Rul H, Van Bael MK, Mullens J, Van Poucke LC (2007) J Mater Sci 42:624
Truijen I, Van Bael MK, Van den Rul H, D’Haen J, Mullens J (2007) J Sol-Gel Sci Technol 41:43
Jolivet J-P, Henry M, Livage J (2000) Metal oxide chemistry and synthesis—From solution to solid state. John Wiley & Sons
Mühlebach J, Müller K, Schwarzenbach G (1970) Inorg Chem 9:2381
Lobmann P (2005) J Sol-Gel Sci Technol 33:275
Narendar Y (1996) PhD Thesis, Pennsylvania State University, University Park, Pennsylvania
Colthup NB, Daly LH, Wiberley SE (1990) Introduction to infrared and Raman spectroscopy. , Academic Press, San Diego
Schumb WC, Satterfield CN, Wentworth RL (1955) Hydrogen peroxide. Reinhold Publishing Corporation, New York
Djordjevic C, Lee M, Sinn E (1989) Inorg Chem 28:719
Dakanali M, Kefalas ET, Raptopoulou CP, Terzis A, Voyiatzis G, Kyrikou I, Mavromoustakos T, Salifoglou A (2003) Inorg Chem 42:4632
Lever ABP, Gray HB (1978) Acc Chem Res 11:348
Hardy A, Vanhoyland G, Geuzens E, Van Bael MK, Mullens J, Van Poucke LC, D’Haen J (2005) J Sol-Gel Sci Technol 33:283
Nakamoto K (1997) Infrared and Raman Spectra of inorganic and coordination compounds part b: applications in coordination, organometallic, and bioinorganic chemistry. John Wiley & Sons, Inc., New York
Deacon GB, Phillips RJ (1980) Coord Chem Rev 33:227
Griffith WP (1964) J Chem Soc 5248
Griffith WP, Wickins TD (1967) J Chem Soc A 590
Werner PE, Eriksson L, Westdahl M (1985) J Appl Crystallogr 18:367
Boultif A, Louer D (1991) J Appl Crystallogr 24:987
Kakihana M, Tada M, Shiro M, Petrykin V, Osada M, Nakamura Y (2001) Inorg Chem 40:891
Greenwood NN, Earnshaw A (1984) Chemistry of the elements. Pergamon Press
Sorrell TN (1988) Interpreting spectra of organic molecules. University Science Books, Mill Valley
Lee TA (1998) A beginner’s guide to mass spectral interpretation. Wiley
Fischer J, Merwin LH, Nissan RA (1995) Appl Spectrosc 49:120
Hardy A, Van Werde K, Vanhoyland G, Van Bael MK, Mullens J, Van Poucke LC (2003) Thermochim Acta 39:143
McDevitt NT, Baun WL (1964) Spectrochim Acta 20:799
JCPDS (1997) Powder diffraction file of inorganic phases, Joint Committee on Powder Diffraction Standards, Swarthmore
Acknowledgements
A Hardy and M.K. Van Bael are postdoctoral research fellows of the Research Foundation—Flanders (FWO Vlaanderen). The authors thank dr. G. Vanhoyland for the (HT-)XRD work and would like to express their gratitude towards late prof. dr. em. L.C. Van Poucke for many fruitful discussions of the results presented here.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hardy, A., D’Haen, J., Van Bael, M.K. et al. An aqueous solution–gel citratoperoxo–Ti(IV) precursor: synthesis, gelation, thermo-oxidative decomposition and oxide crystallization. J Sol-Gel Sci Technol 44, 65–74 (2007). https://doi.org/10.1007/s10971-007-1601-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-007-1601-3