Abstract
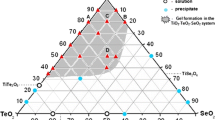
Sol-gel processing of tellurium oxide has been investigated in the tellurium isopropoxide/citric acid/isopropanol/water system. As evidenced by Fourier transformed infrared spectroscopy (FTIR), citric acid has been found to be a relevant chemical modifier to control hydrolysis-condensation reactions of highly reactive tellurium isopropoxide Te(OCH(CH3)2)4. Thus, depending on the main synthesis chemical parameters such as alkoxide concentration, water and modifier ratios, colloidal sols and gels have been successfully synthesised. The thermal behaviour of the dried gels has been investigated by X-ray diffraction, differential scanning calorimetry coupled with thermogravimetry and also FTIR spectroscopy. On the one hand, the crystallisation of the non-centrosymmetric γ-TeO2 polymorph as well as the α-TeO2 phase which the crystallite size ranges from a few ten nanometers (∼50 nm) to a few microns as a function of heat treatment, and, on the other hand, the synthesis of homogeneous sols which can be handled in air and so particularly suitable for the elaboration of thin films provide new opportunities for making tellurite based materials and thin film devices for practical applications.
Similar content being viewed by others
References
Hall DW, Newhause MA, Borelli NF, Dumaugh WH, Weidman DL (1988) Appl Phys Lett 54:1293
El-Mallawany RAH (2001) Tellurite glasses handbook: physical properties and data. CRC Press, Boca Raton, Florida
Kim S-H, Yoko T, Sakka S (1993) J Am Ceram Soc 76:2486
Jeansannetas B, Blanchandin S, Thomas P, Marchet P, Champarnaud-Mesjard JC, Merle-Mejean T, Frit B, Nazabal V, Fargin E, Le Flem G, Marti MO, Bousquet B, Canioni L, Le Boiteux S, Segonds P, Sarger L (1999) J Sol St Chem 146:329
Stegeman R, Rivero C, Richardson K, Stegeman G, Delfyett Jr P, Guo Y, Pope A, Schulte A, Cardinal T, Thomas P, Champarnaud-Mesjard JC (2005) Opt Express 13:1144
Pierre A, Duboudin F, Tanguy B, Portier J (1992) J Non-Cryst Solids 147&148:569
Hodgson SNB, Weng L (2000) J Non-Cryst Solids 276:195
Weng L, Hodgson SNB (2001) Mater Sci Eng B87:77
Weng L, Hodgson SNB (2002) J Non-Cryst Solids 297:18
Weng L, Hodgson SNB (2002) Opt Mat 19:313
Doeuff S, Henry M, Sanchez C, Livage J (1987) J Non-Cryst Solids 89:206
Coste S, Thomas P, Lecomte A, Champarnaud-Mesjard J-C, Guinebretière R, Dauger A (2002) Phys Chem Glasses 43C:405
Coste S (2003) Thesis of the University of Limoges, France
Coste S, Lecomte A, Thomas P, Champarnaud-Mesjard JC, Merle-Mejean T, Guinebretière R (2004) J Non-Cryst Solids 345&346:634
Pechini M (1967) U.S. Patent, n°3 330 697 (July 11th 1967)
Pechini M (1966) U.S. Patent, n°3 231 328 (January 25th 1966)
Masson O (1998) Thesis of the University of Limoges, France
Alcock NW, Tracy VM, Waddington TC (1976) J Chem Soc Dalton Trans 2243
Mehrotra RC, Bohra R (1983) Metal carboxylates. Academic Press, London
Deacon GB, Phillips RJ (1980) Coord Chem Rev 33:227
Tsay JD, Fang T-T (1999) J Am Ceram Soc 82(6):1409
Rajendran M, Subba Rao M (1994) J Solid State Chem 113:239
Alcock NW, Tracy VM, Waddington TC (1976) J Chem Soc Dalton Trans 2243
Mehrotra RC, Bohra R (1983) Metal carboxylates. Academic Press, London, p 48
Lecomte A, Lenormand P, Dauger A (2000) J Appl Cryst 33:496
Dutreilh-Colas M (2001) Thesis of the University of Limoges, France
Masson O, Guinebretière R, Dauger A (1998) Mat Sci Forum 278–281, Proc. of the 5th EPDIC, 115
Langford JI (1978) J Appl Cryst 11:10
Langford JI (1992) In: Prince E, Stalick JK (eds) Accuracy in Powder Diffraction II. NIST special publication, 846 Washington, p 110
Williamson GK, Hall WH (1953) Acta Metall 1:22
Lecomte A, Bamière F, Coste S, Thomas P, Champarnaud-Mesjard JC (in press) J Eur Ceram Soc
Vrillet G, Lasbrugnas C, Thomas P, Masson O, Couderc V, Barthélémy A, Champarnaud-Mesjard JC (2005) J Mat Sci 40:4975
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Coste, S., Lecomte, A., Thomas, P. et al. Sol-gel synthesis of TeO2-based materials using citric acid as hydrolysis modifier. J Sol-Gel Sci Technol 41, 79–86 (2007). https://doi.org/10.1007/s10971-006-0117-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-006-0117-6