Abstract
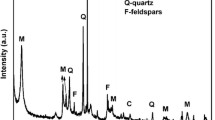
The effect of humic acid (HA) and competing cations Na+ and Ca2+ on the sorption of uranium and europium onto bentonite was studied by batch experiments in aqueous solutions. Sorption isotherms were obtained and simulated satisfactorily by Langmuir and Freundlich models and thermodynamic parameters (ΔH0, ΔS0 and ΔG0) were calculated. The results showed that the sorption was significantly depended on pH, initial concentration, sorbent mass, presence of HA and competing cations and temperature and found to increase in the presence of HA. Several techniques such as Fourier Transform Infrared spectroscopy, X-ray powder diffraction, Scanning Electron Microscopy as well as determination of BET-surface area were applied to support the investigation.











Similar content being viewed by others
References
Abdel Rahman RO, Ibrahium HA, Hung Y-T (2011) Liquid radioactive wastes treatment: a review. Water 3(2):551–565
Misaelides P (2019) Clay minerals and zeolites for radioactive waste immobilization and containment: a concise overview. In: Mercurio M, Sharkar B, Langella A (eds) Modified clay and zeolite nanocomposite materials: environmental and pharmaceutical applications, Chapter 10. Elsevier pp 243–274
Abou-Lilah RA, Rizk HE, Elshorbagy MA, Gamal AM, Ali AM, Badawy NA (2020) Efficiency of bentonite in removing cesium, strontium, cobalt and uranium ions from aqueous solution: encapsulation with alginate for column application. J Environ Anal Chem. https://doi.org/10.1080/03067319.2020.1761348
Runde W (2000) The chemical interactions of actinides in the environmment. Los Alamos Sci 26:392–411
Noli F, Fedorcea V, Misaelides P, Gretescu I, Kapnisti M (2021) Cesium and barium removal from aqueous solutions in the presence of humic acid and competing cations by a Greek bentonite from Kimolos Island. Appl Radiat Isot 170:109600
Wang J, Chen Z, Shao D, Lic Y, Xu Z, Cheng C, Asirid AM, Marwanid HM, Hu S (2017) Review adsorption of U(VI) on bentonite in simulation environmental conditions. J Mol Liq 242:678–684
Toxic Substances Portal-Uranium. Toxicological Profile for Uranium (2013) Agency for Toxic Substances and Disease Registry. US Department of Health and Human. https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=440&tid=77
Karpas Z (2015) Analytical chemistry of uranium. Environmental, forensic, nuclear and toxicological applications. CRC Press Taylor & Francis group
Noli F, Kapnisti M, Buema G, Harja M (2016) Retention of barium and europium radionuclides from aqueous solutions on ash-based sorbents by application of radiochemical techniques. Appl Radiat Isot 116:102–109
He Y, Chen Y, Ye W, Zhang X (2020) Effects of contact time, pH, and temperature on Eu (III) sorption onto MX-80 bentonite. Chem Phys 534:110742
Nakashima Y (2006) H2O self-diffusion coefficient of water-rich MX80 bentonite gels. Clay Miner 41:659–668
Hurel C, Marmier N (2010) Sorption of europium on a MX-80 bentonite sample: experimental and modelling results. J Radioanal Nucl Chem 284:225–230
Vaidyanathan G (2013) Nuclear Reactor Engineering (Principle and Concepts). S. Chand & Company Pvt. Ltd., New Delhi
Ren X, Wang S, Yang S, Li J (2009) Influence of contact time, pH, soil humic/fulvic acids, ionic strength and temperature on sorption of U(VI) onto MX–80 bentonite. J Radioanal Nucl Chem 283:253–259
Youssef WM (2017) Uranium adsorption from aqueous solution using sodium bentonite activated clay. J Chem Eng Process Technol 8:1–8
Xiao J, Chen Y, Zhao W, Xu J (2013) Sorption behavior of U(VI) onto Chinese bentonite: Effect of pH, ionic strength, temperature and humic acid. J Mol Liq 188:178–185
Tan L, Wang X, Tan X, Mei H, Chen C, Hayat T, Alsaedi A, Wen T, Lu S, Wang X (2017) Bonding properties of humic acid with attapulgite and its influence on U(VI) sorption. Chem Geol 464:91–100
Li S, Wang X, Huang Z, Du L, Tan Z, Fu Y, Wang X (2015) Sorption and desorption of uranium(VI) on GMZ bentonite: effect of pH, ionic strength, foreign ions and humic substances. J Radioanal Nucl Chem 308:877–886
Hu J, Zhi X, Bo H, Sheng G, Chen C, Li J, Chen Y, Wang X (2010) Sorption of Eu(III) on GMZ bentonite in the absence/presence of humic acid studied by batch and XAFS techniques. Sci China Chem 53:1420–1428
Herbert H, Moog H (1999) Cation exchange, interlayer spacing, and water content of MX-80 bentonite in high molar saline solutions. Eng Geol 54:55–65
Savvin S (1964) Analytical applications of arsenazo III—II: determination of thorium, uranium, protactinium, neptunium, hafnium and scandium. Talanta 11:1–6
Freundlich H (1906) Adsorption in solution. Phys Chem Soc 40:1361–1368
Liu Y, Liu YJ (2008) Biosorption isotherms, kinetics and thermodynamics. Sep Purif Technol 61:229–242
Lagergren S (1898) Zur theorie der sogenannten adsorption geloesterstoffe. Kungl Svenska Vetenskapsakad Handl 24:1–39
Toxicity characteristic leaching procedure. Washington, DC (1992) https://www.epa.gov/hw-sw846/sw-846-test-method-1311-toxicity-characteristic-leaching-procedure. Accessed 30 Mar 2021
MEDUSA (Make Equilibrium Diagrams Using Sophisticated Algorithms) (1983) I. Puigdomènech, INPUT, SED, and PREDOM: computer programs drawing equilibrium diagrams. Report TRITA-OOK-3010, Royal Institute of Technology (KTH), Dept. Inorg. Chemistry, Stockholm
Bradbury MH, Baeyens B (2002) Sorption of Eu on Na- and Ca-montmorillonites: experimental investigations and modelling with cation exchange and surface complexation. Geochim Cosmochim Acta 66(13):2325–2334
Bachmaf S, Planer-Friedrich B, Merkel BJ (2008) Effect of sulfate, carbonate, and phosphate on the uranium(VI) sorption behavior onto bentonite. Radiochim Acta 96:359–366
Kapnisti M, Noli F, Misaelides P, Vourlias G, Karfaridis D, Hatzidimitriou A (2018) Enhanced sorption capacities for lead and uranium using titanium phosphates; sorption, kinetics, equilibrium studies and mechanism implication. Chem Eng J 342:184195
Lu Z, Hao Z, Wang J, Chen L (2016) Efficient removal of europium from aqueous solutions using attapulgite-iron oxide magnetic composites. J Ind Eng Chem 34:374–381
Wang X, Xu D, Chen ZX, Ren A, Chen C (2006) Sorption and complexation of Eu (III) on alumina: Effects of pH, ionic strength, humic acid and chelating resin on kinetic dissociation study. Appl Radiat Isot 64:414–421
Wang X, Chen Y, Wu Y (2004) Diffusion of Eu(III) in compacted bentonite-effect of pH, solution concentration and humic acid. Appl Radiat Isot 60:963–969
Fox P, Davis J, Zachara J (2006) The effect of calcium on aqueous uranium(VI) speciation and adsorption to ferrihydrite and quartz. Geochim Cosmochim Acta 70:1379–1387
De Pablo L, Chavez M, Abatal M (2011) Adsorption of heavy metals in acid to alkaline environments by montmorillonite and Ca-montmorillonite. Chem Eng J 171:1276–1286
Kautenburger R, Beck H (2010) Influence of geochemical parameters on the sorption and desorption behaviour of europium and gadolinium onto kaolinite. J Environ Monit 12:1295–1301
Awual MR, Kobayashi T, Miyazaki Y, Motokawa R, Shiwaku H, Suzuki S, Okamoto Y, Yaita T (2013) Selective lanthanide sorption and mechanism using novel hybrid Lewis base (N-methyl-N-phenyl-1,10-phenanthroline-2-carboxamide) ligand modified adsorbent. J Hazard Mater 252–253:313–320
Gao Y, Shao Z, Xiao Z (2015) U(VI) sorption on illite: effect of pH, ionic strength, humic acid and temperature. J Radioanal Nucl Chem 303:867–876
Han H, Cheng C, Hu S, Li X, Wang W, Xiao C, Xu Z, Shao D (2017) Facile synthesis of gelatin modified attapulgite for the uptake of uranium from aqueous solution. J Mol Liq 234:172–178
Abukhadra MR, Ali SM, El-Sherbeeny AM, Tawhid A, Soliman A, Abd EAE, Elgawad, (2020) Effective and environmental retention of some radioactive elements (U(VI), Sr(II), and Ba(II)) within bentonite/zeolite hybrid structure; equilibrium and realistic study. Inorg Chem Commun 119:108053
Krajňák A, Viglašová E, Michal Galamboš M, Lukáš Krivosudský L (2018) Kinetics, thermodynamics and isotherm parameters of uranium(VI) adsorption on natural and HDTMA-intercalated bentonite and zeolite. Desalin Water Treat 127:272–281
Yu T, Wu WS, Liu Z, Zhang SW, Nie ZW (2013) Kinetic and thermodynamic study of Eu(III) sorption on natural red earth in South China. Korean J Chem Eng 30(2):440–447
Chen YG, Zhu BH, Wu DB, Wang QG, Yang YH, Ye WM, Guo JF (2012) Eu(III) adsorption using di(2-thylhexly) phosphoric acid-immobilized magnetic GMZ bentonite. Chem Eng J 181–182:387–396
Bhattacharyya KG, Gupta SS (2011) Removal of Cu(II) by natural and acid-activated clays: An insight of adsorption isotherm, kinetic and thermodynamics. Desalination 272(1–3):66–75
Ibrahim HA, Abdel Moamen OA, Abdel Monem N, Ismail IM (2018) Assessment of kinetic and isotherm models for competitive sorption of Cs+ and Sr2+ from binary metal solution onto nanosized zeolite. Chem Eng Com 205(9):1274–1287
Acknowledgement
The authors would like to thank Professor E. Pavlidou (Physics Dept., Aristotle Univ.) for the SEM/EDS examination of the samples.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Noli, F., Papalanis, E., Tsamos, P. et al. The effect of humic acid and competing cations Na+ and Ca2+ on the sorption of uranium and europium, onto bentonite from Kimolos Island (Greece). J Radioanal Nucl Chem 328, 1231–1241 (2021). https://doi.org/10.1007/s10967-021-07722-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-07722-y