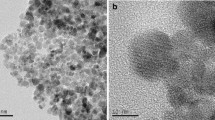
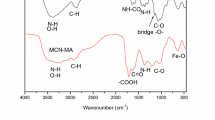
Abstract
The performance of a cross-linked magnetic chitosan has been investigated for the adsorption of U(VI) from aqueous solutions. The influences of the pH of solution, the initial concentration of U(VI) ions and the contact time on the adsorption amounts have been discussed, and the appropriate process conditions for the adsorption of U(VI) have been obtained. The adsorption experimental equilibrium data was found to fit the Langmuir model well, and the uptake of U(VI) was 161.3 mg g−1. Two simplified models including pseudo-first order and pseudo-second order equations are selected to follow the adsorption process.










Similar content being viewed by others
References
Bampaiti A, Misaelides P, Noli F (2014) Uranium removal from aqueous solutions using a raw and HDTMA-modified phillipsite-bearing tuff. J Radioanal Nucl Chem. doi:10.1007/s10967-014-3796-4
Semiao AJC, Rossiter HM, Schafer AI (2010) Impact of organic matter and speciation on the behaviour of uranium in submerged ultrafiltration. J Membr Sci 348:174–180
Daher AM, Wanees SA, Kellah HMA, Ali AH (2014) Removal of uranium from sulfate leach liquor of salcrete depositsusing tri-n-octyl amine. J Radioanal Nucl Chem 299:493–499
Gao MW, Zhu GR, Wang XH, Wang P, Gao CJ (2014) Preparation of shorts channels SBA-15-PVC membrane and its adsorption properties for removal of uranium(VI). J Radioanal Nucl Chem. doi:10.1007/s10967-014-3862-y
Zhang YG, Chi HJ, Zhang WH, Sun YY, Liang Q, Gu Y, Jing RY (2014) Highly efficient adsorption of copper ions by a PVP-reduced graphene oxide based on a new adsorptions mechanism. Nano Micro Lett 6(1):80–87
Modrzejewska Z, Sujka W, Dorabialska M, Zarzycki R (2006) Adsorption of Cr(VI) on cross-linked chitosan beads. Sep Sci Technol 41:111–122
Cestari AR, Vieira EFS, Oliveira IA, Bruns RE (2006) The removal of Cu (II) and Co (II) from aqueous solutions using cross-linked chitosan-Evaluation by the factorial design methodology. J Hazard Mater 143:8–16
Chang YC, Chang SW, Chen DH (2006) Magnetic chitosan nanoparticles: studies on chitosan binding and adsorption of Co(II) ions. React Funct Polym 66:335–341
Hisen TY, Rorrer GL (1995) Effect of acylation and cross-linking on the material properties and cadium ion adsorption capacity of porous chitosan beads. Sep Sci Technol 30:2455–2475
Martinez L, Agnely F, Leclerc B, Siepmann J, Cotte M, Deiger S, Couarraze G (2007) Cross-linking of chitosan and chitosan poly(ethylene oxide)beads: a theoretical treatment. Eur J of Pharm Biopharm 67:339–348
Hisen TY, Rorrer GL, Way JD (1993) Synthesis of porous-magnetic chitosan beads for removal of cadmium ions from wastewater. Ind Eng Chem Res 32:2170–2178
Li JP, Song LM, Zhang SJ (2002) Rare earth metal ion adsorption capacity on crosslinked magnetic chitosan. J Chin Soc Rare Earths 20:219–221 (In Chinese)
Wang CL, Li Y, Liu CL (2015) Sorption of uranium from aqueous solutions with graphene oxide. J Radioanal Nucl Chem 304(3):1017–1025
Hadjittofi L, Pashalidis I (2015) Uranium sorption from aqueous solutions by activated biochar fibres investigated by FTIR spectroscopy and batch experiments. J Radioanal Nucl Chem 304(2):897–904
Meng H, Li Z, Ma FY, Jia LN, Wang XN (2015) Preparation and characterization of surface imprinted polymer for selective sorption of uranium(VI). J Radioanal Nucl Chem. doi:10.1007/s10967-015-4067-8
Acknowledgments
This research was financially supported by the Natural Science Foundation of Jiangxi Province, China (No. 20132BAB203019).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, G., Zou, L., Su, Y. et al. Adsorption of uranium(VI) from aqueous solutions using cross-linked magnetic chitosan beads. J Radioanal Nucl Chem 307, 1135–1140 (2016). https://doi.org/10.1007/s10967-015-4275-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4275-2