Abstract
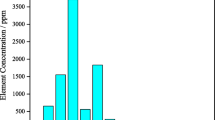
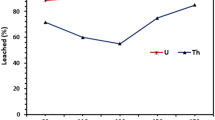
This study was carried-out to leach uranium from rock phosphate using sulphuric acid in the presence of potassium chlorate as an oxidant and to investigate the relative purity of different forms of yellow cakes produced with ammonia, magnesia and sodium hydroxide as precipitants, as well as purification of the products with TBP and matching its impurity levels with specifications of the commercial products. Alpha-particle spectrometry was used for determination of activity concentration of uranium isotopes in rock phosphate, resulting phosphoric acid, and in different forms of the yellow cake. Likewise, atomic absorption spectroscopy was used for determination of impurities. On the average, the equivalent mass concentration of uranium was 119.38 ± 79.66 ppm (rock phosphate) and 57.85 ± 20.46 ppm (phosphoric acid) with corresponding low percent of dissolution (48 %) which is considered low. The isotopic ratio (234U:238U) in all stages of hydrometallurgical process was not much different from unity indicating lack of fractionation. Upon comparing the levels of impurities in different form of crude yellow cakes, it was found that the lowest levels were measured in hydrated trioxide (UO3·xH2O). This implies that saturated magnesia is least aggressive relative to other precipitants and gives relatively pure crude cake. Therefore, it was used as an index to judge the relative purity of other forms of yellow cakes by taking the respective elemental ratios. The levels of impurities (Fe, Zn, Mn, Cu, Ni, Cd and Pb) in the purified yellow cake were found comparable with those specified for commercial products.

Similar content being viewed by others
References
Habashi Fathi (1962) Econ Geol 57(7):1081–1084
Weterings K, Janssen J (1985) Hydrometallurgy 15:173–190
Lottering MJ, Lorenzen L, Phala NS, Smit JT, Schalkwyk GAC (2008) Miner Eng 21:16–22
Singh DK, Mishra SL, Singh H (2006) Hydrometallurgy 81:214–218
Puzikov EA, Zilberman B Ya, Fedorov S Yu, Blazheva IV (2013) Radiochemistry 55(3):291–297
Zhang C, Dodge CJ, Malhotra SV, Francis AJ (2013) Bioresour Technol 136:752–756
Mellah A, Chegrouche S, Barkat M (2007) Hydrometallurgy 85:163–171
Joshi JM, Pathak PN, Pandey AK, Manchand VK (2009) Hydrometallurgy 96:117–122
Singh Abhilash S, Mehta KD, Kumar V, Pandey BD, Pandey VM (2009) Hydrometallurgy 95:70–75
Singh Suman Kumar, Dhami PS, Tripathi SC, Dakshinamoorthy A (2009) Hydrometallurgy 95:170–174
IAEA (1993) Uranium extraction technology, Technical report series No. 359, Vienna (1993)
Morais CA, Gomiero LA, Scassiotti Filho W, Rangel Jr. H (2005) Minerals Engineering 18:1331–1333
Mageed AA (1998) Sudan industrial minerals and rocks. Khartoum, Sudan
Bouwer EJ, Mcklveen JW, McDowel WJ (1978) Health Phys. 34: 345–352
Brinck JW (1974) (Proc. Symo. On the formation of uranium ore deposits, Athens, 6–10 May 1974). International Atomic Energy Agency, Vienna
Rout A, Venkatesan KA, Srinivasan TG, Vasudeva Rao PR (2012) Hazard Mater 221–222:62–67
Cecal A, Humelnicu D, Rudic V, Cepoi L, Ganju D, Cojocari A (2012) Bioresour Technol 118:19–23
Kolchin IV, Gedeonov AD, Vlasov Yu G, Avenirov AK (2012) Radiochemistry 54(4):353–357
Acknowledgments
I would like to thank my colleagues at Environmental Monitoring of Radioactivity Lab, Sudan Atomic Energy Commission for their help during the practical work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
AbowSlama, E.H.Y., Ebraheem, E. & Sam, A.K. Precipitation and purification of uranium from rock phosphate. J Radioanal Nucl Chem 299, 815–818 (2014). https://doi.org/10.1007/s10967-013-2703-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-013-2703-8