Abstract
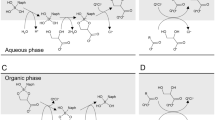
In the present paper, N,N,N’,N’-tetraoctyl diglycolamide (TODGA) as the extractant and n-dodecane as the diluent, the extraction kinetics behavior of Am(III) in TODGA/n-dodecane–HNO3 system were studied, including stirring speed, the interfacial area, extractant concentration in n-dodecane, extracted ions concentration, acidity of aqueous phase and temperature. The results show that: the extraction process is controlled by diffusion mode under 130 rpm of stirring speed and by chemical reaction mode above 150 rpm. The extraction rate equation and the apparent extraction rate constant of Am(III) by TODGA/n-dodecane in 170 rpm and at 25 °C are followed as:








Similar content being viewed by others
References
Modolo G, Vijgen H (2003) Demonstration of the TODGA process for partitioning of actinides(III) from high level liquid waste. Proceedings of the international workshop on P&T and ADS development the Belgian Nuclear Research Centre, in Mol, 6–8 October, pp. 1–9
Nash KL, Lumetta GJ (2011) Advanced separation techniques for nuclear fuel reprocessing and radioactive waste treatment. Woodhead Publishing Series in Energy, 2
Ozawa M, Koma Y, Nomura K et al (1998) Separation of actinides and fission products in high-level liquid wastes by the improved TRUEX process. J Alloys Compd 271–273:538–543
Rizvi GH, Mathur JN (2002) Stripping of actinides, fission and corrosion products from loaded TRUEX solvent (0.2 M CMPO + 1.2 M TBP in dodecane) phase using potassium ferrocyanide. J Radioanal Nucl Chem 253(1):179–181
Zhu YJ, Jiao RZ (1994) Chinese experience in the removal of actinides from highly active waste by trialkylphosphine oxide extraction. Nucl Technol 108(3):361–369
Morita Y, Glatz JP, Kubota et al (1996) Actinide partitioning from HLW in a continuos DIDPA extraction process by means of centrifugal extractors. Solv Extr Ion Exch 14(3):385–400
Watanabe M, Tatsugae R, Morita Y et al (2002) Back-extraction of tri- and tetravalent actinides from diisodecylphosphoric acid (DIDPA) with hydrazine carbonate. J Radioanal Nucl Chem 252(1):53–57
Serrano-Purroy D, Baron P, Christiansen B et al (2005) Recovey of minor actinides from HLLW using DIAMEX process. Radiochim Acta 93(6):351–355
Hérès X, Lanoë JY, Borda G et al. (2009) Counter-current tests to demonstrate the feasibility of extractant separation in diamex-sanex process. Proceedings of Global 2009 Paris, France, 6–11 September, 2009. Paper 9199
Narita H, Yaita T, Tamura K et al (1998) Solvent extraction of trivalent lanthanoid ions with N,N’-dimethyl-N,N’-diphenyl- 3-oxapentanediamide. Radiochim Acta 81:223
Narita H, Yaita T, Tamura K et al (1999) Study on the extraction of trivalent lanthanide ions with N, N’-dimethyl-N,N’-diphenyl-malonamide and -diglycolamide. J Radioanal Nucl Chem 239:381
Sasaki Y, Sugo Y, Suzuki S et al (2001) The novel extractants, diglycolamides, for the extraction of lanthanides and actinides in HNO3-n-dodecane system. Solv Extr Ion Exch 19:91
Tachimori S, Sasaki Y, Suzuki S (2002) Modification of TODGA n-dodecane solvent with a monoamide for high loading of lanthanides (III) and actinides(III). Solv Extr Ion Exch 20:687
Panja S, Mohapatra PK, Tripathi SC et al (2012) A highly efficient solvent system containing TODGA in room temperature ionic liquids for actinide extraction. Sep Purif Technol 96:289–295
Mohapatraa PK, Iqbalb M, Raut DR et al (2011) Evaluation of a novel tripodal diglycolamide for actinide extraction:solvent extraction and SLM transport studies. J Membr Sci 375:141–149
Ansari SA, Pathak PN, Husain M et al (2006) Extraction of actinides using N, N, N’, N’-tetraoctyl diglycolamide (TODGA): a thermodynamic study. Radiochim Acta 94:37–312. doi:10.1524/ract.2006.94.6.307
Morita Y, Sasakki Y, Tachimori S (2002–2004) Actinide separation by TODGA extraction. JAERI-Conf 2002–2004:255–260
Magnusson D, Christiansen B, Glatz J-P et al. (2007) Partitioning of minor actinides from purex raffinate by the TODGA Process. Proceedings of GLOBAL 2007[C]. Boise, Idaho, USA, 9–13 Sept 2007, pp. 713–718
Modolo G, Asp H, Vijgen H et al. (2007) Demonstration of a TODGA/TBP process for recovery of trivalent actinides and lanthanides from a purex raffinate. Proceedings of GLOBAL 2007[C]. Boise, Idaho, USA, 9–13 Sept 2007, pp. 1111–1116
Dutta S, Mohapatra PK, Manchand VK (2011) Separation of 90Y from 90Sr by a solvent extraction method using N,N,N’,N’-tetraoctyl diglycolamide (TODGA) as the extractant. Appl Radiat Isot 69:158–162
Lou Z-N, Xiong Y, Song J-J et al (2010) Kinetics and mechanism of Re(VII) extraction and separation from Mo(VI) with trialkylamine. Trans Nonferrous Met Soc China 20(2010):s10–s14
Lewis JB (1954) The mechanism of mass transfer of solutes across liquid–liquid interfaces. Chem Eng Sci 3:248–259
Danesi PR, Chiarzia R (1980) The kinetics of metal solvent extraction. In: Campbell B (ed) Critical reviews in analytical chemistry, vol 10(1). CRC Press, Boca Raton, p 5
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, WB., Ye, GA., Li, FF. et al. Kinetics and mechanism of Am(III) extraction with TODGA. J Radioanal Nucl Chem 298, 1749–1755 (2013). https://doi.org/10.1007/s10967-013-2653-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-013-2653-1