Abstract
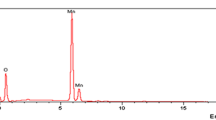
Nanocrystalline manganese oxide was prepared and characterized using various techniques like XRD, surface area analyzer and zeta potential measurements. The sorption characteristics with respect to uptake of various ions including uranyl have been evaluated. Various experimental conditions which affect the sorption characteristics have been studied. Nanocrystalline manganese oxide was prepared by the hydrolysis of KMnO4 and the nano oxide were found to have a size of 8 nm and surface area of 145 m2/g. Due to the high surface area, the sorption property of the nano oxide was good. It was found that the sorption was achieved at different pH values and with varying time of equilibration. Thus it is seen that the kinetics was an important aspect for the possible separation of metal ions.










Similar content being viewed by others
References
Bhagyashree K, Mishra RK, Shukla R, Kasar S, Kar A, Kumar S, Kumar S, Kaushik CP, Tyagi AK, Tomar BS (2012) J. Radioanal Nucl Chem. doi:10.1007/s10967-012-1938-0
Ozeroglu C, Metin N (2012) J Radioanal Nucl Chem 292:923
Zhao DL, Yang SB, Chen SH, Guo ZQ, Yang X (2011) J Radioanal Nucl Chem 287:557
Kamei-Ishikawa N, Tagami K, Uchida S (2007) J Radioanal Nucl Chem 274(3):555
Petroni SLG, Pires MAF, Munita CS (2004) J Radioanal Nucl Chem 259(2):239
Sadeghi M, Sarabadani P, Karami H (2010) J Radioanal Nucl Chem 283:297
Sadeghi M, Karami H, Sarabadani P, Bolourinovin F (2009) J Radioanal Nucl Chem 281:619
Varma PCR, Manna RS, Banerjee D, Varma MR, Suresh KG, Nigam AK (2008) J Alloys Compd 453:298
Dong W, Wu S, Chen D, Jiang X, Zhu C (2000) Chem Lett 5:496
Baratto C, Lottici PP, Bersani D, Antonioli G, Gnappi G, Montenero A (1998) J Sol–Gel Sci Technol 13:667
Albertina C, Martyn P (2001) J Mater Sci 11:1408
Grimm S, Schultz M, Barth S, Muller R (1997) J Mater Sci 32:1083
Fukumasa O, Fujiwara T (2003) Thin Solid Films 435:33
Zhao SY, Lee DK, Kim CW, Cha HG, Kim YH, Kang YS (2006) Bull Korean Chem Soc 27:237
Paike VV, Niphadkar PS, Bokade VV, Joshi PN (2007) J Am Ceram Soc 90:3009
Falqui A, Musinu A, Cannas C, Peddis D, Piccaluga G (2006) J Nanopart Res 8:255
Bhaduri S, Bhaduri SB (2008) Ceram Int 28:153
Yuan J, Laubernds K, Villegas J, Gomez S, Suib SL (2004) Adv Mater 16:1729
Brock SL, Sanabria M, Suib SL, Urban V, Thiyagarajan P, Potter DI (1999) J Phys Chem B 103:7416
Brock SL, Duan NG, Tian ZR, Giraldo O, Zhou H, Suib SL (1998) Chem Mater 10:2619
Ramkumar Jayshree, Shukla R, Chandramouleeswaran S, Mukherjee T, Tyagi AK (2012) Nanosci Nanotechnol Lett 4:1
Qin Li, Yunhai L, Xiaohong C, Cui P (2012) J Radioanal Nucl Chem 293:67
Zheng-ji Y, Jun Y (2012) J Radioanal Nucl Chem 293:907
Faghihian H, Peyvandi S (2012) J Radioanal Nucl Chem 293(3):783
Xiaoliang W, Guowen P, Yan Y, Wang Y, Tingting H (2012) J Radioanal Nucl Chem 291(3):825
Konstantinou M, Pashalidis I (2007) J Radioanal Nucl Chem 273(3):549
Hongxia Z, Chao Y, Zuyi T (2007) J Radioanal Nucl Chem 279(1):317
Baik MH, Hyun SP, Hahn PS (2003) J Radioanal Nucl Chem 256(1):11
Chandramouleeswaran S, Ramkumar J, Sudarsan V, Reddy AVR (2011) J Hazard Mater 198:159
Langmuir I (1918) J Am Chem Soc 40:1361
Saeed MM, Rusheed A, Ahmed N (2005) J Radioanal Nucl Chem 211(2):283
Atun G, Kilislioglu A (2003) J Radioanal Nucl Chem 258(3):605
Freundlich HMF (1906) J Phys Chem 57:385
Abd El-Rahman KM, El-Kamash AM, El-Sourougy MR, Abdel-Moniem NM (2006) J Radioanal Nucl Chem 268(2):221
Dubinin MM (1960) Chem Rev 60:235
Nayak D, Lahiri S (2006) J Radioanal Nucl Chem 267:59
Zou WH, Zhao L, Zhu L (2012) J Radioanal Nucl Chem 292:1303
Ng JCY, Cheung WH, McKay G (2002) J Colloid Interface Sci 255:64
Gimbert F, Morin-Crini N, Renault F, Badot PM, Crini G (2008) J Hazard Mater 157:34
Shih-Chin T, Kai-Wei J (2000) J Radioanal Nucl Chem 243(3):741
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Mukherjee, J., Ramkumar, J., Chandramouleeswaran, S. et al. Sorption characteristics of nano manganese oxide: efficient sorbent for removal of metal ions from aqueous streams. J Radioanal Nucl Chem 297, 49–57 (2013). https://doi.org/10.1007/s10967-012-2393-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-012-2393-7