Abstract
137Cs activity concentrations were determined in samples of macrophytes Polysiphonia fucoides (red algae) and Zostera marina (vascular plant) collected during the entire vegetation season in the Gulf of Gdańsk in the southern Baltic Sea. The measurements showed considerable seasonality of 137Cs activity in both species; an increase of cesium concentrations was observed from spring to autumn with maximal levels 49.1 ± 1.4 Bq kg −1d.w. (P. fucoides) and 14.5 ± 1.0 Bq kg −1d.w. (Z. marina) in late autumn. 137Cs concentrations observed in a given season are the result of a number of processes, the intensity of which can differ depending on external environmental conditions. The effects of these processes can differ and their directions can frequently be opposite to one another. The examined macrophytobenthic plant species could serve as bioindicators of radionuclide pollution for monitoring purposes on condition that the samples of plants are taken within a strictly defined period of the year to give comparable results and to supply realistic information about pollution levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of benthic plants as bio-indicators for monitoring surveys of environmental pollutants is widely recommended by many authors [1–5]. Macrophytobenthic organisms are also investigated as potential reference organisms to be used in models for assessing the radiological consequences of radioactive releases, arising both from routine operation of nuclear facilities [6] and from accidental situations [7]. The main reason for such applications is their well known ability to accumulate trace elements like heavy metals and radionuclides [5, 8–12]. The main advantage of using seaweeds and vascular plants as indicators of radionuclides is their way of exchanging elements with the environment [13]. In the case of macroalgae the exchange is accomplished through the entire surface of the thalli, so-called foliar uptake, while in the case of vascular plants it is accomplished mainly via the root system. Additionally, in the case of benthic plants, the response of a given bioindicator occurs relatively quickly after the pollution event [14]. In contrast to benthic fauna or fish, which accumulate hazardous substances as long as they live, the level of radionuclides in benthic plants can reflect changes of radionuclide concentrations observed in seawater during a single year of vegetation.
The rationale for using seaweeds as indicators of radionuclide contamination also includes the fact that contaminant concentrations in environmental solutions (e.g. seawater) are often close to the analytical detection limits and often vary with time. Seaweeds, accumulating metals from solution, can integrate short-term temporal fluctuations in concentration [13].
Bioaccumulative processes are determined by external and internal factors [13]. The external factors are temperature, light conditions, salinity and the concentration of the investigated trace element, in both its physical and chemical form. Internal factors are connected with the morphology, physiology and habitat of the investigated organisms [13].
The life history of a plant, which may be defined as a continuous interaction between the plant and its biotic or abiotic environment, is a significant factor with regards to the bioaccumulation process as the life cycle also depends on both the internal and external factors. A more widespread mechanism of the life cycle is that of following circa-annual rhythms as a means of timing growth [13], but seaweeds can also (or instead) respond to environmental cues. The most important and obvious cues are temperature and light and the switch from vegetative growth to reproduction (which in most seaweeds involves very little growth) often depends on them [13]. Some plants are more sensitive to temperature and others to light conditions.
Besides the development phase and age of the studied plant, intensity of vital processes is another parameter directly related to seasonality. Changing thermal conditions, light conditions and concentrations of various substances (e.g. nutrients) all affect the bioaccumulation ability [13]. The seasonal variability of trace metal concentrations has been observed in Zostera marina and Ulva rigida [11] and, in the latter case the concentrations of Cu and Cd seemed at least partially to reflect growth dynamics [3, 15]. Low concentrations were found in spring, when the plant biomass reached its maximum. The increase in the biomass led to a decrease in the metal content due to dilution [16]. The diluting effect on radionuclide concentrations caused by growth could be an even more significant parameter than the biological loss of radionuclides [17].
The complexity of bioaccumulation in benthic plants and the multitude of factors affecting this process are the main causes of the wide range of radionuclide concentrations observed in the plants and, as a consequence, in concentration coefficients determined by various authors regarding specific radionuclides or plant species [6]. Therefore, the results obtained have to be interpreted with considerable caution and it must be noted that studies concerning the effects of particular factors on bioaccumulation process are in great demand, especially in an environmental context.
This paper presents the results of a study of radionuclide bioaccumulation in marine plants during an entire vegetation period, the aim of which is to examine seasonal changes in the activity of 137Cs in selected plant species and to determine to what degree the biological activity and developmental phase influence the bioaccumulation process. The selection of radioactive 137Cs was prompted by the fact that it is a nuclide of solely anthropogenic origin and its activities in marine matrices in the Baltic Sea are much higher than those of other artificial isotopes [18–22]; its half-life is relatively long at 30.05 years [23]. The majority of 137Cs in the Baltic Sea was deposited as a result of the accident at the Chernobyl nuclear power plant in 1986. It is estimated that the overall load of 137Cs that reached the sea from this source was 4.7 TBq, comprising 82% of the total amount of 137Cs that has accumulated in the Baltic seawater [19, 24]. For this reason, the Baltic Sea is still considered the most polluted marine area in terms of 137Cs [25].
Materials and methods
Sampling
The study was conducted on Polysiphonia fucoides, a species of the red algae division as well as Z. marina, representing vascular plants. The selected species are characteristic of the flora of the southern Baltic Sea and can be used as potential bioindicators of the marine environment pollution.
Benthic plants were collected in the Gulf of Gdańsk in the southern Baltic Sea, at one location called Kępa Redłowska at definite depth of 2–3 m (Fig. 1). Sampling was conducted by a scuba diver who collected the plants from the seafloor. At each depth the metal frame of 0.5 × 0.5 m was deployed three times and the ensuing subsamples were integrated, the results were then related to the stretch of 0.75 m2 (3 × 0.25 m2). Integrated samples immersed in plastic bags were transported to the laboratory, where further preparation was conducted. The sampling was performed to follow the seasonal cycle of plant development in November 2008, in January, in February, in March, in April, in May and in July 2009; the macrophyte community at this depth was comprised mainly of P. fucoides and Z. marina.
Seawater samples were taken during the monitoring cruises onboard R/V ‘Baltica’ in June 2008 and 2009. Sampling was carried out at stations located in the Gulf of Gdańsk area (Fig. 1) from the surface layer, bottom (2 m above the seabed) and down the water profile (at ZN4 and P1 stations) with the rosette sampler (General Oceanics, model 1015). Salinity and temperature were measured simultaneously along the sampling profile using sonda CTD MARK III (Neil Brown Instrument System Inc.).
Temperature and salinity data from 2006 to 2007 are the results of systematic monitoring in the area of Kępa Redłowska. They were measured with Valeport 602 sonda CTD (in 2006) and with Idronaut 304 sonda CTD (in 2007).
Sample preparation
The plant material was analyzed taxonomically, individual species occurring in the samples were identified and biomass of each species was determined gravimetrically; taxonomic analyses and biomass determination were carried out according to the HELCOM COMBINE guidelines [26]. For the radionuclide activity determination, the selected plant species were dried, ashed at 450 °C and homogenized. Ashed samples were transferred in counting boxes of the appropriate shape and size.
137Cs activity concentration in seawater samples was measured by two methods. In 2008, the radiochemical method was applied and preparation of samples was following a procedure described in Zalewska and Lipska [22]. Non-filtered seawater samples of water of approx. 30 dm3 were acidified to pH 1–2 with hydrochloric acid and 20 mg of a natural carrier of cesium was added. After equilibration, 10 g of ammonium phosphomolybdate (AMP) was added and the content was stirred for 20 min. After 24 h the liquid phase was decanted and separated AMP was dissolved in NaOH. Then cesium was re-precipitated on 1 g of AMP, re-dissolved in NaOH and purified in a column filled with Bio-Rex 40 cation-exchange resin. Following separation of the accompanying ions (NH4 +, Na+, K+ and Rb+), cesium was eluted with 3 M hydrochloric acid. The obtained eluate was evaporated to dryness, digested and dissolved in dilute hydrochloric acid. Finally cesium was precipitated as chloroplatinate and its recovery was estimated gravimetrically. β-Radiation of samples was measured using Low-Level Beta Counter FHT 7700T (ESM Eberline) with the background count rate of 0.01 counts s−1 and the minimum detectable activity of 3 mBq per sample. The measurement time for beta radiation analysis was 7,200 s.
In 2009, 137Cs activity was determined by gamma spectrometry method. As an initial step 20 mg Cs+ was added to each acidified seawater sample as a carrier. Cesium was absorbed on 10 g of AMP during 20 min stirring. The separated (by decantation and filtration) AMP was dried and measured using gamma spectrometry system.
Analysis and measurement
137Cs content in samples of biological material and in seawater samples taken in 2009 was measured by gamma spectrometry method using an HPGe detector with a relative efficiency of 18% and a resolution of 1.8 keV for peak of 1332 keV of 60Co. The detector was coupled with an 8192-channel computer analyser and GENIE 2000 software. The measurement time for gamma spectrometry was 80,000 s.
The reference solution: “Standard solution of 137Cs, code SRCs-23, IM/25/O/00”, activity 4.2 kBq, total uncertainty ±1.5%, referenced date 9.11.2000, produced at the IBJ-Swierk k/Otwocka Poland, was used for preparing reference samples for the equipment calibration. Different measurements geometries were applied in isotope determination of seawater and plant samples. The respective reference samples were prepared in identical geometries as in the relevant matrix.
The reliability and accuracy of the applied method was verified by participation in the inter-comparison exercises organized by IAEA-MEL Monaco (IAEA-414, Irish and North Sea Fish) [27]. Repeated analysis gave a value equal to 5.06 ± 0.64 Bq kg −1d.w. while the estimated target value was equal to 5.18 ± 0.10 Bq kg −1d.w. .
Results and discussion
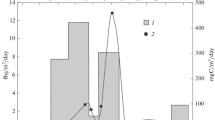
Changes in the 137Cs concentration of P. fucoides and Z. marina, collected systematically at depth of 2–3 m between July 2008 and October 2009, are shown in Fig. 2. The changes show similar character although a significant difference in the level of isotope accumulation can be observed, with much higher 137Cs concentrations determined in P. fucoides across the entire study period. Differences in 137Cs activity in relation to time and sampling location were also observed in the case of red algae by Nonova and Strezov [28]. Comparatively lower concentrations of 137Cs, observed in vascular plant Z. marina (Fig. 2), when compared with P. fucoides, may be linked to the method by which elements are exchanged between these benthic plants and their environment. The foliar uptake which is characteristic of algae makes bioaccumulation of cesium ions more effective taking into account the exchange surface area and additionally the water surrounding the plant provides a continuous source of the radionuclide. Vascular plants, on the other hand, can exchange elements with their environment both through root systems embedded in sand and through leaves. The contribution of these two exchange pathways in the general uptake depends strongly on the element availability, i.e. concentration. There is evidence that leaf tissues have higher nutrient uptake affinities than root tissues at low-element availability [29]. However, in general the pore water shows higher nutrients concentration, which could be also the case of cesium and for this reason it could be expected that the main way of this isotope exchange is through the root system.
An increase in the content of radioactive cesium was observed in both plant species from July until the end of the year, more intensive in the case of P. fucoides. The maximal concentrations measured in P. fucoides and Z. marina were 49.1 ± 1.4 Bq kg −1d.w. and 14.5 ± 1.0 Bq kg −1d.w. in November 2008 and January 2009 respectively. Simultaneously, seawater temperature was decreasing and found its minimal level in February—Fig. 3. The data presented here for 2006 and 2007 show seasonal pattern of seawater temperature and salinity that is common for the investigated area. The subsequent warming up of the marine environment was not accompanied by an increase in 137Cs content in the plants; on the contrary, its concentrations decreased further. The minimum 137Cs concentration in Z. marina (1.7 ± 0.3 Bg kg −1d.w. ) was measured in March (2009). In the case of P. fucoides the minimum concentration (12.5 ± 1.0 Bq kg −1d.w. ) was found in April (2009). In May 2009 cesium activity increased again to 20.8 ± 2.5 and 4.5 ± 1.9 Bq kg −1d.w. in P. fucoides and Z. marina respectively. 137Cs activity increased in the following months in P. fucoides and its activity level determined in July 2009 (23.8 ± 3.9 Bq kg −1d.w. ) did not differ from that in July 2008 (24.2 ± 1.4 Bq kg −1d.w. ). The similar patterns of seasonal changes observed in both plants suggests that bioaccumulation processes are mainly determined by the physiological activity connected directly with environmental factors. Similar observation have been made for the heavy metals Pb, Cu, Zn and Fe in Enteromorpha linza, concentrations of which followed the same pattern with the minimum observed in April and maximum values observed in October and January [30].
Radionuclide concentrations observed in the studied benthic plants were related solely to the plant morphology and physiology, those having close relation to environmental conditions like temperature and sunlight, and not to the changes of 137Cs in seawater. Spatial distribution of 137Cs in the Gulf of Gdańsk, examined at four locations showed a homogenous pattern of radionuclide concentrations (Fig. 4). No significant differences were noted between 2008 and 2009, either. Monitoring measurements (in 2006 and 2007) of seawater temperature and salinity in the area close to the macrophyte sampling region showed strong seasonality in the surface seawater temperature changes (Fig. 3). Simultaneously, temperature remained quite uniform in the water column on given dates (Fig. 5). Salinity remained stable along the vertical profile (Fig. 6) and showed minor seasonal response (Fig. 3). The uniform vertical distribution of temperature and salinity provide evidence for the strong mixing trough waves and advection, in the examined area and hence imply that 137Cs concentrations will be uniform down the water column profile.
The life history of the Polysiphonia genus is quite a complicated process [31, 32]. In the vegetatively favourable period, during the spring and summer, an intense increase in biomass of Polysiphonia is being observed. In the winter time, the biomass is considerably reduced and Polysiphonia creates spore forms called tetraspore and delivered by tetrasporophyte. Small male and female plants, called early gametophyte, are formed from tetraspre. These forms start to grow during the spring to create gametophyte and tetrasporophyte, which are recognized as young forms of benthic plants. They mature during the summer and autumn [13]. From April to September, several phases of reproductive cycle can generally be observed but, as expected, the young plants dominate at the beginning of this cycle. It is a well known fact that the lowest concentrations of metals usually occur in the growing part of a plant, and that higher, more constant values are recorded in the older tissue [11, 33]. It has been explained in the case of Fucus vesiculosus by relatively slow accumulation of metals in the young parts of plants, as well as by synthesis of a greater number of binding sites in older tissue [34]. It has also been shown in Elodea canadensis for example that element (241Am) distribution varies depending on the age of the leaf blades and the state of the cells [33].
The curves which characterise seasonal changes of 137Cs in P. fucoides and Z. marina tissues may indicate a relationship between external environmental factors, such temperature and light conditions, and the life cycle of the plants. In summer, under optimal growth conditions, intensive plant growth and fruiting result in an increase of biomass. These growth processes are directly related to metabolic processes: photosynthesis and respiration. Simultaneously, as the intensification of metabolic processes results in enhanced metal uptake, the augmentation of biomass causes a diluting effect. Therefore, the final cesium concentrations in the plants in summer can be seen to be the result of these two opposing processes (Fig. 7). In autumn, as in summer, there are two opposite tendencies which affect cesium content in the plant tissues. Metabolic processes are still rather intensive and enhance bioaccumulation but, on the other hand, the decline in temperature and sunlight conditions, acts against the metabolic activity and slows it down. This slowing down causes a decrease in the rate of bioaccumulation and is also seen in the biomass decline, although the latter effect can be considered as a concentration process (opposite to the diluting process connected with the increment of biomass). The final effect, resulting from these opposed activities, is the continued increase of 137Cs concentration in plant tissue in autumn. In winter, when the loss of biomass is very intensive due to plant fading, cesium ions can be released/resuspended in the water and hence their concentration in plant cells declines. This process generates an additional source of 137Cs in the marine environment. Young plants that appear at the turn of the year and in spring contain low amounts of 137Cs mainly because of the short time of bioaccumulation.
Conclusions
-
1.
A strong seasonality in 137Cs bioaccumulation in macrophyte species P. fucoides and Z. marina was detected. 137Cs concentrations increased in both plants from spring to autumn, reaching their maximum values in late autumn.
-
2.
The intensity of 37Cs bioaccumulation varied depending on the physiological activity of the plants, i.e. intensity of synthesis and respiration processes which in turn were affected by environmental conditions. The final 37Cs concentration in the plant tissue was a result of these processes which can have opposed influence.
-
3.
Z. marina, a vascular plant showed lower 137Cs concentrations as compared to P. fucoides indicating worse efficiency of the bioaccumulation process in vascular plant. However, the pattern of seasonal changes observed in both plants in the entire investigation period was very similar indicating that bioaccumulation processes are mainly determined by the physiological activity connected directly with environmental factors.
-
4.
P. fucoides can be recommended as a good bioindicator for monitoring of 37Cs pollution on account of the level of isotope concentrations during the study period, the available biomass along the depth profile and the occurrence throughout the entire vegetation season. At the same time, however, the application of plant bioindicators for monitoring purposes has to be done with particular caution as the time of year exerts a strong influence on the analysed element concentrations.
References
Burger J, Gochfeld M, Kosson DS, Powers CW, Jewett S, Friedlander B, Chenelot H, Volz CD, Jeitner C (2006) Radionuclides in marine macroalgae from Amchitka and Kiska Islands in the Aleutians: establishing a baseline for future biomonitoring. J Environ Radioact 91:27–40
Kleinschmidt R (2009) Uptake and depuration of 131I by the macroalgae Catenella nipae—potential use as an environmental monitor for radiopharmaceutical waste. Mar Pollut Bull 58:1539–1543
Malea P, Haritonidis S (2000) Use of the green alga Ulva rigida C. Agardh as an indicator species to reassess metal pollution in the Thermaikos Gulf, Greece, after 13 years. J. Appl. Phycol. 12:169–176
Strezov A, Nonova T (2005) Radionuclide accumulation in green and brown macroalgae at the Bulgarian Black Sea coast. Environ Monit Assess 265(1):21–29
Strezov A, Nonova T (2009) Influence of macroalgal diversity on accumulation of radionuclides and heavy metals in Bulgarian Black Sea ecosystem. J Environ Radioact 100:144–150
Kumblad L, Kautsky U, Naesslund B (2006) Transport and fate of radionuclides in aquatic environments—the use of ecosystem medelling for exposure assessments of nuclear facilities. J Environ Radioact 87:107–129
Lepicard S, Heling R, Maderich V (2004) POSEIDON/RODOS models for radiological assessment of marine environment after accidental releases: application to costal areas of the Baltic, Black and North Seas. J Environ Radioact 72:153–161
Al-Masri MS, Mamish S, Budier Y (2003) Radionuclides and trace metals in eastern Mediterranean Sea algae. J Environ Radioact 67:157–168
Sawidis T, Heinrich G, Brown M-T (2003) Cesium-137 concentration in marine macroalgae from different biotopes in the Aegean Sea (Greece). Ecotoxicol Environ Saf 54:249–254
Skwarzec B, Ulatowski J, Strumińska DI, Falandysz J (2003) Polonium 210Po in the phytobenthos from Puck Bay. J Environ Monit 5:308–311
Szefer P (2002) Metal pollutants and radionuclides in the Baltic Sea—an overview. Oceanologia 44:129–178
Wolterbeek H Th, Viragh A, Sloof JE, Bolier G, van der Veer B, de Kok J (1995) On the uptake and release of zinc (65Zn) in the growing alga Selenastrum capricornutum Printz. Environ Pollut 88:85–90
Lobban CS, Harrison PJ (1997) Seaweed ecology and physiology. Cambridge University Press, New York. ISBN-13 978-0-521-40897-4
Boisson F, Hutchins DA, Fowler SW, Fisher NS, Teyessie J-L (1997) Influence of temperature on the accumulation and retention of 11 radionuclides by marine alga Fucus vesiculosus. Mar Pollut Bull 35:313–327
Haritonidis S, Malea P (1995) Seasonal and local variation of Cr, Ni and Co concentrations in Ulva rigida C. Agardh and Enteromorpha linza (Linnaeus) from Thermaikos Gulf, Greece. Environ Pollut 89:319–327
Malea P, Haritonidis S, Stratis I (1994) Bioaccumulation of metals by Rhodophyta species at Antikyra Gulf (Greece) near an aluminium factory. Bot Mar 37:505–513
Dahlgaard H, Boelskifte S (1992) ‘Sensi’: a model describing the accumulation and time integration of radioactive discharges in the bioindicator Fucus vesiculosus. J Environ Radioact 16:49–63
HELCOM (1995) Radioactivity in the Baltic Sea 1992–1998. In: Baltic Sea environment proceedings, no. 61, pp 59–68
HELCOM (2003) Radioactivity in the Baltic Sea 1992–1998. In: Baltic Sea environment proceedings, no. 85, pp 5–15
HELCOM (2009) Radioactivity in the Baltic Sea 1999–2006. In: Baltic Sea environment proceedings, no. 117, pp 47–49
Ikaheimonen TK, Outola I, Vartti V-P, Kotilainen P (2009) Radioactivity in the Baltic Sea: inventories and temporal trends of 137Cs and 90Sr in water and sediments. J Radioanal Nucl Chem 282(2):419–425
Zalewska T, Lipska J (2006) Contamination of the southern Baltic Sea with 137Cs and 90Sr over the period 2000–2004. J Environ Radioact 91:1–14
Bé M-M, Chisté V, Dulieu C, Browne E, Baglin C, Chechev VP, Egorov A, Kuzmenko NK, Sergeev VO, Kondev FG, Luca A, Galán M, Huang X, Wang B, Helmer RG, Schönfeld E, Dersch R, Vanin VR, de Castro RM, Nichols AL, MacMahon TD, Pearce A, Lee KB, Wu SC (2008) Monographie BIPM-5, tables of radionuclides (comments on evaluation), vol 1–4. Bureau International des Poids et Measures, Sevres
Nielsen SP, Bengston P, Bojanowski R, Hagel P, Herrmann J, Ilus E, Jakobson E, Motiejunas S, Panteleev Y, Skujina A, Suplinska M (1999) The radiological exposure of man from radioactivity in the Baltic Sea. Sci Total Environ 237(238):133–141
IAEA (2005) Worldwide marine radioactivity studies—WOMARS, Radionuclides levels in Oceans and Sea, IAEA-TECDOC-1429. ISBN 92-0-114904-2
HELCOM (1997) Manual for marine monitoring in the COMBINE programme of HELCOM. http://sea.helcom.fi/Monas/CombineManual2/CombineHome.htm
IAEA (2010) HELCOM-MORS proficiency test determination of radionuclides in fish flesh sample, IAEA/AQ/13. ISSN 2047-7659
Nonova T, Strezov A (2005) Radionuclide uptake in red macroalgae from the Bulgarian Black Sea coast. J Radioanal Nucl Chem 266(3):411–417
Larkum AWD, Orth RJ, Duarte CM (2006) Seagrasses: biology, ecology and conservation. Springer, Netherlands. ISBN-10-4020-2942-X(HB)
Malea P, Haritonidis S (1999) Metal content in Enteromorpha linza (Linnaeus) in Thermaikos Gulf (Greece). Hydrobiologia 394:103–112
Kim M-S, Lee IK, Boo SM (1994) Morphological studies of the red alga Polysiphonia morrowii Harvey on the Korean Coast. Korean J Phycol 9(2):185–192
Szweykowska A, Szweykowski J (1979) Botanika. PWN, Warszawa (in Polish)
Bondareva L, Vlasova I, Mogilnaya O, Bolsunovsky A, Kalmykov S (2010) Microdistribution of 241Am in structures of submerged macrophyte Elodea canadensis growing in the Yenisei River. J Environ Radioact 101:16–21
Bryan GW, Langston WJ, Hummerstone LG, Burt GR (1985) A guide to the assessment of heavy-metal contamination in estuaries using biological indicators. Marine Biological Association of the United Kingdom, Occasional Publication. The Laboratory, Plymouth, p 4
Acknowledgments
The author would like to thank Michał Saniewski and express gratitude for the help in collection of phytobenthos samples used in this study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Zalewska, T. Seasonal changes of 137Cs in benthic plants from the southern Baltic Sea. J Radioanal Nucl Chem 292, 211–218 (2012). https://doi.org/10.1007/s10967-011-1546-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-011-1546-4