Abstract
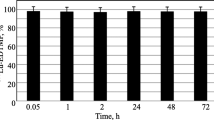
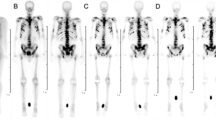
The radiopharmaceutical [153Sm]Sm-EDTMP is administered for painful bone metastases with the standard dosage of 37 MBq/kg, without evaluation of patient individual characteristics. For a better dose estimate in vivo stability should be considered, because labelled and unlabelled samarium do not have the same metabolic pathway. We evaluated radiopharmaceutical in vitro stability, measuring the activity by beta and gamma spectrometry. Subsequently we verified in vivo stability on serial blood and urine samples. The percentage of the unlabelled radiopharmaceutical is high and, on the basis of radiochemical data as well as blood clearance and urine excretion, we calculated the main parameters for a preliminary biokinetic model.




Similar content being viewed by others
References
De Vita VT Jr, Helman S, Rosemberg SA (1993) Cancer, principles and practice of oncology. Lippincott, Philadelphia-USA
Willis RW (1978) Tumours: basic principles and clinical aspects. Longman, New York
Serafini AN (2001) J Nucl Med 42:895
Abramson SD, Weissman G (1989) Clin Exp Rheumatol 7:163
Wolkert WA, Goeckeler WF, Ehrhardt GJ, Ketring AR (1991) J Nucl Med 32:174
Stöcklin G, Qaim SM, Rösch F (1995) Radiochim Acta 70/71:249
McEwan AJB (2000) Semin Radiat Oncol 10:103
Cameron PJ, Klemp PFB, Martindale AA, Turner JH (1999) Nucl Med Commun 20:609
Farhanghi M, Holmes RA, Volkert WA, Logan KW, Singh A (1992) J Nucl Med 33:1451
Brenner W, Kampen WU, Von Forstner C, Brümmer C, Zuhayra M, Muhle C, Czech N, Henze E (2001) J Nucl Med 42:1545
Annals of International Commission on Radiological Protection (ICRP) (1981) Publication 30, Part 3 including Addendum to Parts 1 and 2
Gasiglia HT, Okada H (1995) J Radioanal Nucl Chem 199:29
Shargel L, Yu ABC (1980) Applied biopharmaceutics and pharmacokinetics. Appleton-Century-Crofts, New York
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ridone, S., Arginelli, D., Inglese, E. et al. Evaluation of in vitro and in vivo stability of the radiopharmaceutical [153Sm]Sm-EDTMP for biokinetics studies in bone metastases pain palliation care. J Radioanal Nucl Chem 282, 287–291 (2009). https://doi.org/10.1007/s10967-009-0277-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-009-0277-2