Abstract
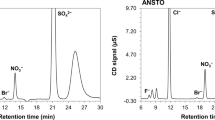
In the present exploratory study, the applicability of anionic impurities for attributing nuclear material to a certain chemical process or origin has been investigated. Anions (e.g., nitrate, sulphate, fluoride, chloride) originate from acids or salt solutions that are used for processing of solutions containing uranium or plutonium. The study focuses on uranium ore concentrates (“yellow cakes”) originating from different mines. Uranium is mined from different types of ore body and depending on the type of rock, different chemical processes for leaching, dissolving and precipitating the uranium need to be applied. Consequently, the anionic patterns observed in the products of these processes (the “ore concentrates”) are different. The concentrations of different anionic species were measured by ion chromatography using conductivity detection. The results show clear differences of anion concentrations and patterns between samples from different uranium mines. Besides this, differences between sampling campaigns in a same mine were also observed indicating that the uranium ore is not homogeneous in a mine. These within-mine variations, however, were smaller than the between-mine variations.
Similar content being viewed by others
References
M. Peehs, T. Walter, S. Walter, Uranium, Uranium Alloys, and Uranium Compounds, Ullmann’s Encyclopedia of Industrial Chemistry, Vol. A27.
M. Wallenius, K. Mayer, G. Tamborini, A. Nicholl, Intern. Conf. on Advances in Destructive and Non-Destructive Analysis for Environmental Monitoring and Nuclear Forensics, 21–23 October 2002, Karlsruhe, Germany.
E. Keegan, S. Richter, I. Kelly, H. Wong, P. Gadd, H. Kühn, A. Alonso-Munoz, Appl. Geochem., 23 (2008) 765.
J. Svedkauskaite-Legore, G. Rasmussen, S. Abousahl, P. Van Belle, J. Radioanal. Nucl. Chem. (online), DOI: 10.1007/s10967-007-7215-y.
L. Pajo, K. Mayer, L. Koch, Fresenius J. Anal. Chem., 371 (2001) 348.
G. L. Long, J. D. Winefordner, Anal. Chem., 55 (1983) A712.
V. Hostomská, J. Hostomský, J. Hazard. Mater., 147 (2007) 342.
http://www.cameco.com/operations/uranium/rabbit_lake/mining_and_milling.php
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Badaut, V., Wallenius, M. & Mayer, K. Anion analysis in uranium ore concentrates by ion chromatography. J Radioanal Nucl Chem 280, 57–61 (2009). https://doi.org/10.1007/s10967-008-7404-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-008-7404-3