Abstract
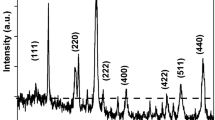
In order to investigate the physical and chemical effects of neutron capture reaction, a neutron in-beam Mössbauer spectroscopic study on two isomorphs of iron disulfide: pyrite and marcasite, were carried out with a parallel plate avalanche counter at room temperature. In both compounds only two major products accounted for the obtained spectrum: one with Mössbauer parameters close to the parent compound and the other one considered to be a new product. The yield of the parent-like species was different in the two isomorphs.
Similar content being viewed by others
References
T. Tominaga, E. Tachikawa, Modern Hot Atom Chemistry and Its Applications, Springer, Berlin, Heidelberg, New York, 1981.
T. Matsuura, Hot Atom Chemistry: Recent Trends and Applications in the Physical and Life Sciences and Technology, Kodansha, Tokyo, and Elsevier, Amsterdam, 1984.
H. Sano, Hot-Atom Chemistry and Trapped Species, in: Chemical Mössbauer Spectroscopy, R. H. Herber (Ed.), Plenum Press, New York, 1984, p. 177.
Y. Kobayashi, Y. Yoshida, M. K. Kubo, Y. Yamada, A. Yoshida, H. Ogawa, H. Ueno, K. Asahi, Eur.Phys. J., A13 (2002) 243.
Y. Kobayashi, M. K. Kubo, Y. Yamada, T. Saito, H. Ueno, H. Ogawa, W. Sato, K. Yoneda, H. Watanabe, N. Imai, H. Miyoshi, K. Asahi, J. Radioanal. Nucl. Chem., 255 (2003) 403.
W. G. Berger, J. Fink, F. E. Obenshain, Phys. Lett., 24A (1967) 466.
W. G. Berger, Z. Phys., 225 (1969) 139.
G. Czjzek, W. G. Berger, Phys. Rev., B1 (1970) 957.
M. K. Kubo, Y. Kobayashi, Y. Yamada, Y. Nemoto, T. Saito, Y. Sakai, H. Shoji, C. Yonezawa, H. Matsue, M. Nakada, AIP Conf. Ser., 715 (2005) 348.
C. Yonezawa, K. K. H. Wood, M. Hoshi, Y. Ito, E. Tachikawa, Nucl. Instr. Meth. Phys. Res., A329 (1993) 207.
S. L. Finklea III, L. Cathey, E. L. Amma, Acta Cryst., 32A (1976) 529.
J. R. Smyth, T. C. McCormick, Crystallographic Data for Minerals, in: Mineral Physics and Crystallography: A Handbook of Physical Constants, the American Geophysical Union, 1995.
K. Ono, A. Ito, E. Hirahara, J. Phys. Soc. Japan, 17 (1962) 1615.
S. S. Hafner, B. T. Evans, G. M. Kalvius, Solid State Commun., 5 (1967) 17.
J. A. Morice, L. C. Rees, D. T. Ricard, J. Inorg. Nucl. Chem., 31 (1969) 3797.
C. A. Taft, S. F. Chuha, N. G. Souza, N. C. Futado, J. Phys. Chem. Solids, 41 (1980) 61.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kobayashi, Y., Tsuruoka, Y., Kubo, M.K. et al. Mössbauer spectroscopic study of 57Fe species produced by 56Fe(n,γ)57Fe reaction in iron disulfide. J Radioanal Nucl Chem 272, 623–626 (2007). https://doi.org/10.1007/s10967-007-0635-x
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10967-007-0635-x