Abstract
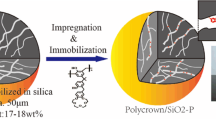
A novel macroporous silica-based 2,6-bis(5,6-diisobutyl-1,2,4-triazine-3-yl)pyridine (iso-Bu-BTP), a neutral chelating agent having several softatom nitrogen, polymeric composite (iso-Bu-BTP/SiO2-P) was synthesized. It was done through impregnation and immobilization of iso-Bu-BTP molecule into the pores of SiO2-P particles with 40–60 μm of bead diameter and 0.6 μm of mean pore size. The effective impregnation resulted from the intermolecular interaction of iso-Bu-BTP and co-polymer inside the SiO2-P particles by a vacuum sucking technique. To understand the possibility of applying iso-Bu-BTP in the MAREC process developed, the adsorption behavior of a few representative rare earths (REs) such as Ce(III), Nd(III), Gd(III), Dy(III), Er(III), Yb(III), and Y(III) towards iso-Bu-BTP/SiO2-P was investigated at 298 K. The influence of the HNO3 concentration in a wide range of pH 5.52–3.0M and a few chelating agents such as formic acid, citric acid, and diethylenetriaminepentaacetic acid (DTPA) on the adsorption of RE(III) was examined. It was found that in the presence of chelating agent, the adsorption ability of the tested RE(III) towards iso-Bu-BTP/SiO2-P decreased due to two competition reactions of RE(III) with iso-Bu-BTP/SiO2-P and chelating agents. In a 0.01M HNO3 solution containing 1M formic acid or 1M citric acid, light RE(III) showed lower adsorption towards iso-Bu-BTP/SiO2-P than that of the heavy one. This makes the separation of light RE(III) from the heavy one possible. Based on the similarity of minor actinides and heavy RE(III) in chemical properties and the results of column separation experiments, chromatographic partitioning of light RE(III) from a simulated high level liquid waste solution composed of the heavy RE(III) and minor actinides in MAREC process is promising.
Similar content being viewed by others
References
E. D. Collins, J. P. Renier, Systems studies of actinide patitioning-transmutation recycle methods, Proc. Intern. Conf. Global 2005, Tsukuba, Japan, October 9–13, 2005, p. 235.
C. Madic, Overview of the hydrometallurgical and pyromdallurgical processes studied world-wide for the partitioning of high active nuclear wastes, Proc. 3th Intern. Conf. NUCEF 2001, Japan Atomic Energy Research Institute, Tokai-mura, Ibaraki, Japan, 31 October–2 November 2001, JAERI Conf 2002–004, March 2002, p. 27.
G. R. Choppin, K. L. Nash, Radiochim. Acta, 70–71 (1995) 225.
J. M. Mathur, M. S. Murali, K. L. Nash, Solvent Extr. Ion Exch., 19 (2001) 357.
Y. Morita, Y. Sasaki, S. Tachimori, Actinides separation by TODGA extraction, Proc. 3th Intern. Conf. NUCEF 2001, Japan Atomic Energy Research Institute, Tokai-mura, Ibaraki, Japan, 31 October–2 November 2001, JAERI Conf 2002–004, March 2002, p. 255.
K. L. Nash, P. G. Rickert, E. P. Horwitz, Solvent Extr. Ion Exch., 7 (1989) 655.
U.S. Department of Energy (DOE). TRUEX/SREX Demonstration Innovation Technology Summary Reports, DOE/EM-0419, December, 1998, p. 18.
R. Chiarizia, E. P. Horwitz, Solvent Extr. Ion Exch., 4 (1986) 677.
A. Zhang, Y.-Z. Wei, M. Kumagai, Y. Koma, T. Koyama, Radiat. Phys. Chem., 72 (2005) 455.
A. Zhang, Y.-Z. Wei, M. Kumagai, Y. Koma, T. Koyama, Radiat. Phys. Chem., 72 (2005) 669.
K. L. Nash, R. C. Gatrone, G. A. Clark, P. G. Rickert, E. P. Horwitz, Separ. Sci. Technol., 23 (1988) 1355.
M. L. Dietz, E. P. Horwitz, R. Chiarizia, H. Diamona, Novel extraction chromatographic materials for the separation and preconcentration of radionuclides, Proc. Intern. Solvent Extraction Conference (ISEC’93), University of York, England, 9–15 September, 1993, Vol. 3, p. 1587.
E. P. Horwitz, M. L. Dietz, D. M. Nelson, J. J. Larosa, W. D. Fairman, Anal. Chim. Acta, 238 (1990) 263.
Y.-Z. Wei, A. Zhang, M. Kumagai, M. Watanabe, N. Hayashi, J. Nucl. Sci. Technol., 41 (2004) 315.
A. Zhang, Y.-Z. Wei, M. Kumagai, Y. Koma, A new partitioning process for high level liquid waste by extraction chromatography using impregnated adsorbents, 2003 ANS/ENS Intern. Winter Meeting, Global 2003, New Orleans, Louisiana, November 16–20, 2003.
A. Zhang, Y.-Z. Wei, M. Kumagai, Y. Koma, J. Alloy. Comp., 390 (2005) 275.
A. Zhang, Y.-Z. Wei, M. Kumagai, Y. Koma, J. Radioanal. Nucl. Chem., 269 (2006) 119.
A. Zhang, E. Kuraoka, H. Hoshi, M. Kumagai, J. Chromatogr., A1061 (2004) 175.
A. Zhang, Y.-Z. Wei, M. Kumagai, React. Funct. Polym., 61 (2004) 191.
A. Zhang, Y.-Z. Wei, M. Kumagai, Solvent Extr. Ion Exch., 21 (2003) 591.
A. Zhang, Y.-Z. Wei, H. Hoshi, M. Kumagai, Adsorp. Sci. Technol., 22 (2004) 497.
A. Zhang, Y.-Z. Wei, H. Hoshi, M. Kumagai, Separ. Sci. Technol., 40 (2005) 811.
A. Zhang, Y.-Z. Wei, H. Hoshi, M. Kumagai, Solvent Extr. Ion Exch., 23 (2005) 231.
A. Zhang, Y.-Z. Wei, M. Kumagai, J. Radioanal. Nuch. Chem., 265 (2005) 409.
A. Zhang, E. Kuraoka, M. Kumagai, Separ. Purif. Technol., 50 (2006) 35.
A. Zhang, Y.-Z. Wei, M. Kumagai, T. Koyama, J. Radioanal. Nucl. Chem., 262 (2004) 739.
A. Zhang, Y.-Z. Wei, H. Hoshi, M. Kumagai, Adsorp. Sci. Technol., 23 (2005) 721.
Z. Kolarik, Solvent Extr. Ion Exch., 21 (2003) 381.
Z. Kolarik, U. Müllich, F. Gassner, Solvent Extr. Ion Exch., 17 (1999) 1155.
H. Hoshi, Y.-Z. Wei, M. Kumagai, T. Asakura, Y. Morita, J. Alloy Comp., 408–412 (2006) 1274.
Y.-Z. Wei, M. Kumagai, Y. Takashima, G. Modolo, R. Odoj, Nucl. Technol., 132 (2000) 413.
T. Moeller, L. C. Thompson, J. Inorg. Nucl. Chem., 24 (1962) 499.
R. D. Baybarz, J. Inorg. Nucl. Chem., 27 (1965) 1831.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, A., Kuraoka, E. & Kumagai, M. Preparation of a novel macroporous silica-based 2,6-bis(5,6-diisobutyl-1,2,4-triazine-3-yl)pyridine impregnated polymeric composite and its application in the adsorption for trivalent rare earths. J Radioanal Nucl Chem 274, 455–464 (2007). https://doi.org/10.1007/s10967-006-6923-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-006-6923-z