Abstract
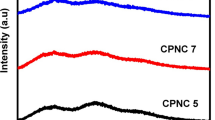
The global issue of environmental pollution has become the motivation for researchers to develop natural based products. Researchers start to substitute synthetic polymers with natural polymers as host and ammonium salts instead of lithium salts for electrolyte application due to biodegradable, safer to handle and low in cost. In this work, a green polymer electrolyte system is prepared by blending 80 wt.% starch and 20 wt.% chitosan with ammonium thiocyanate (NH4SCN) as dopant salt. The highest room temperature conductivity of (1.30 ± 0.34) × 10−4 S cm−1 is obtained when the starch–chitosan blend is doped with 30 wt.% NH4SCN electrolyte which is found to obey Arrhenius rule. The deconvolution of Fourier transform infrared (FTIR) analysis has proved the molecular interaction between starch, chitosan and NH4SCN. The number density (n), mobility (μ) and diffusion coefficient (D) of ions are found to be affected by NH4SCN concentration. This result is further supported by the XRD sample with 30 wt.% NH4SCN which exhibited the most amorphous structure with the lowest degree of crystallinity. Conduction mechanism for the highest conducting electrolyte follows correlated barrier hopping (CBH) model.














Similar content being viewed by others
References
Fan L, Wei S, Li S, Li Q, Lu Y (2018) Recent progress of the solid-state electrolytes for high-energy metal-based batteries. Adv Energy Mater 8:1–31
Li Q, Chen J, Fan L, Kong X, Lu Y (2016) Progress in electrolytes for rechargeable Li-based batteries and beyond. Green energy Environment 1:18–42
Ramesh S, Liew CW, Arof AK (2011) Ion conducting corn starch biopolymer electrolytes doped with ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate. J Non-Cryst Solids 357:3654–3660
Shukur MF, Ibrahim FM, Majid NA, Ithnin R, Kadir MFZ (2013) Electrical analysis of amorphous corn starch-based polymer electrolyte membranes doped with LiI. Phys Scr 88:1–9
Monisha S, Mathavan T, Selvasekarapandian S, Benial AMF, Aristatil G, Mani N, Premalatha M, Pandi DV (2016) Investigation of bio polymer electrolyte based on cellulose acetate-ammonium nitrate for potential use in electrochemical devices. Carbohydr Polym 157:38–47
Aziz SB, Abidin ZHZ (2015) Ion-transport study in nanocomposite solid polymer electrolytes based on chitosan: electrical and dielectric analysis. J Appl Polym Sci 132:1–10
Nirmala Devi G, Chitra S, Selvasekarapandian S, Premalatha M, Monisha S, Saranya J (2017) Synthesis and characterization of dextrin-based polymer electrolytes for potential applications in energy storage devices. Ionics (Kiel) 23:3377–3388
Lin Y, Li J, Liu K, Liu Y, Liu J, Wang X (2016) Unique starch polymer electrolyte for high capacity all-solid-state lithium sulfur battery. Green Chem 18:3796–3803
Chatterjee B, Kulshrestha N, Gupta PN (2016) Nano composite solid polymer electrolytes based on biodegradable polymers starch and poly vinyl alcohol. Measurement 82:490–499
Amran NNA, Manan NSA, Kadir MFZ (2016) The effect of LiCF3SO3 on the complexation with potato starch-chitosan blend polymer electrolytes. Ionics 22:1647–1658
Khanmirzaei MH, Ramesh S, Ramesh K (2015) Polymer electrolyte based dye-sensitized solar cell with rice starch and 1-methyl-3- propylimidazolium iodide ionic liquid. Mater Des 85:833–837
Pokhrel S, Yadav PN, Adhikari R (2015) Applications of chitin and chitosan in industry and medical science. Sci Technol 16:99–104
Manigandan V, Karthik R (2018) Chitosan applications in food industry. Elsevier Inc, India, Chenai, pp 469–491
Zhao D, Yu S, Sun B, Gao S, Guo S, Zhao K (2018) Biomedical applications of chitosan and its derivative nanoparticles. Polymers (Basel) 10:462–479
Aziz SB (2018) The mixed contribution of ionic and electronic carriers to conductivity in chitosan based solid electrolytes mediated by CuNt salt. J Inorg Organomet Polym Mater 28:1942–1952
Aziz SB, Abdullah OG, Al-zangana S (2019) Solid polymer electrolytes based on chitosan:NH4Tf modified by various amounts of TiO2 filler and its electrical and dielectric characteristics. Int J Electrochem Sci 14:1909–1925
Kadir MFZ, Majid SR, Arof AK (2010) Plasticized chitosan-PVA blend polymer electrolyte based proton battery. Electrochim Acta 55:1475–1482
Muthuvinayagam M, Gopinathan C (2015) Characterization of proton conducting polymer blend electrolytes based on PVdF-PVA. Polymer (Guildf) 68:122–130
Mendes JF, Paschoalin RT, Carmona Alfero VB, Sena Neto R, Marques ACP, Marconcini JM, Mattoso LHC, Medeiros ES, Oliveira JE (2015) Biodegradable polymer blends based on cornstarch and thermoplastic chitosan processed by extrusion. Carbohydr Polym 137:452–458
Deshmukh K, Ahamed MB, Reddy A (2016) Impedance spectroscopy , ionic conductivity and dielectric studies of new Li+ ion conducting polymer blend electrolytes based on biodegradable polymers for solid state battery applications. J Mater Sci Mater Electron 27:11410–11424
Yusof YM, Shukur MF, Illias HA, Kadir MFZ (2014) Conductivity and electrical properties of corn starch–chitosan blend biopolymer electrolyte incorporated with ammonium iodide. Phys Scr 89:1–10
Khiar ASA, Arof AK (2011) Electrical properties of starch/chitosan-NH4NO3 polymer electrolyte. Phys Math Sci 5:1662–1666
Hemalatha R, Alagar M, Selvasekarapandian S, Sundaresan B, Moniha V (2019) Studies of proton conducting polymer electrolyte based on PVA, amino acid proline and NH4SCN. J Sci Adv Mater Devices 4:101–110
Nithya S, Selvasekarapandian S, Karthikeyan S (2014) AC impedance studies on proton-conducting PAN:NH4SCN polymer electrolytes. Ionics (Kiel) 20:1391–1398
Aziz NA, Majid SR, Arof AK (2012) Synthesis and characterizations of phthaloyl chitosan-based polymer electrolytes. J Non-Cryst Solids 358:1581–1590
Hemalatha R, Radha KP, Rose JL (2016) AC impedance, FTIR studies of biopolymer electrolyte potato starch:NH4SCN. Multidiscip Educ Res 1:4–6
Buraidah MH, Teo LP, Majid SR, Arof AK (2009) Ionic conductivity by correlated barrier hopping in NH4I doped chitosan solid electrolyte. Phys B 404:1373–1379
Shukur MF, Yusof YM, Zawawi SMM, Illias HA, Kadir MFZ (2013) Conductivity and transport studies of plasticized chitosan-based proton conducting biopolymer electrolytes. Phys Scr T157:1–5
Hamsan MH, Shukur MF, Aziz SB, Kadir MFZ (2019) Dextran from Leuconostoc mesenteroides-doped ammonium salt-based green polymer electrolyte. Bull Mater Sci 3:42–57
Zulkefli FN, Navaratnam S, Ahmad AH (2015) Proton conducting biopolymer electrolytes based on starch incorporated with ammonium thiocyanate. Trans Tech 1112:275–278
Diederichsen KM, Buss HG, Mccloskey BD (2017) The compensation effect in the Vogel− Tammann−Fulcher (VTF) equation for polymer-based electrolytes. Macromolecules 50:3831–3840
Moniha V, Alagar M, Selvasekarapandian S, Sundaresan B, Boopathi G (2018) Conductive bio-polymer electrolyte iota-carrageenan with ammonium nitrate for application in electrochemical devices. J Non-Cryst Solids 481:424–434
Aziz SB, Faraj MG, Abdullah OG (2018) Impedance spectroscopy as a novel approach to probe the phase transition and microstructures existing in CS:PEO based blend electrolytes. Sci Rep 8:1–14
Aziz SB, Woo TJ, Kadir MFZ, Ahmed HM (2018) A conceptual review on polymer electrolytes and ion transport models. J Sci Adv Mater Devices 3:1–17
Karmakar A, Ghosh A (2014) Structure and ionic conductivity of ionic liquid embedded PEO- LiCF3SO3 polymer electrolyte. AIP Adv 4:1–12
Shukur MF, Ithnin R, Kadir MFZ (2014) Electrical properties of proton conducting solid biopolymer electrolytes based on starch–chitosan blend. Ionics (Kiel) 20:977–999
Tamilselvi P, Hema M (2016) Structural, thermal, vibrational and electrochemical behavior of lithium ion conducting solid polymer electrolyte based on poly (vinyl alcohol) / poly (vinylidene fluoride) blend. Poly Sci 58:776–784
Noor NAM, Isa MIN (2019) Investigation on transport and thermal studies of solid polymer electrolyte based on carboxymethyl cellulose doped ammonium thiocyanate for potential application in electrochemical devices. Int J Hydrog Energy 44:1–9
Premalatha M, Mathavan T, Selvasekarapandian S, Mary FK, Umamaheswari R (2016) Characterization of proton conducting blend polymer electrolyte using PVA-PAN doped with NH4SCN. Solid State Physics 1731:1–4
Kadir MFZ, Hamsan MH (2017) Green electrolytes based on dextran-chitosan blend and the effect of NH4SCN as proton provider on the electrical response studies. Ionics 24:2379–2398
Yusof YM, Illias HA, Shukur MF, Kadir MFZ (2016) Characterization of starch-chitosan blend-based electrolyte doped with ammonium iodide for application in proton batteries. Ionics (Kiel) 23:681–697
Kadir MFZ, Aspanut Z, Majid SR, Arof AK (2011) FTIR studies of plasticized poly (vinyl alcohol)–chitosan blend doped with NH4NO3 polymer electrolyte membrane. Spectrochim Acta Part A Mol Biomol Spectroscl 78:1068–1074
Farghali J, Zainal B, Ahmad AH, Farghali J, Zainal B, Hanom A (2015) Conductivity study and fourier transform infrared (FTIR) characterization of methyl cellulose solid polymer electrolyte with sodium iodide conducting ion. Am Inst Phys 020026:1–5
Taylor P, Vicentini NM, Dupuy N, Leitzelman M, Cereda MP (2013) Prediction of cassava starch edible film properties by chemometric analysis of infrared spectra properties by chemometric analysis. Spectrosc Lett 38:749–767
Woo HJ, Majid SR, Arof AK (2011) Conduction and thermal properties of a proton conducting polymer electrolyte based on poly (ε -caprolactone). Solid State Ionics 199–200:14–20
Ramya CS, Selvasekarapandian S (2014) Spectroscopic studies on ion dynamics of PVP– NH4SCN polymer electrolytes. Ionics:3–8
Rahman NAA, Navaratnam S, Abidin SS, Latif FA (2018) FTIR study on the effect of free ions in PMMA/ENR 50 polymer electrolyte system. AIP Conference 020076:1–6
Arya A, Sharma AL (2018) Structural, electrical properties and dielectric relaxations in Na+ ion conducting solid polymer electrolyte. J Phys 30:1–57
Ahmed HT, Abdullah OG (2020) Impedance and ionic transport properties of proton-conducting electrolytes based on polyethylene oxide/methylcellulose blend polymers. J. Sci. Adv. Mater. Devices 20:1–26
Arya A, Sharma AL (2019) Dielectric relaxations and transport properties parameter analysis of novel blended solid polymer electrolyte for sodium-ion rechargeable batteries. J Mater Sci 54:7131–7155
Ramlli MA, Ikmar M, Mohamad N (2016) Structural and ionic transport properties of protonic conducting solid biopolymer electrolytes based on carboxymethyl cellulose doped ammonium fluoride. J Phys Chem B 120:11567–11573
N. H. Muhamaruesa, M. Ikmar (2017) Studies of ionic conductivity and A.C. conduction mechanism of 2-hydroxyethyl cellulose based solid polymer electrolytes. Sustain. Sci. Manag. 65–70
Selvalakshmi S, Vijaya N, Selvasekarapandian S, Premalatha M (2017) Biopolymer agar-agar doped with NH4SCN as solid polymer electrolyte for electrochemical cell application. Appl Polym Sci 44702:1–10
Adnan SBRS, Mohamed NS (2014) Electrical properties of novel Li4.08Zn0.04Si0.96O4 ceramic electrolyte at high temperatures. Ionics (Kiel) 20:1641–1650
Iqbal MZ, Rafiuddin (2015) Structural, electrical conductivity and dielectric behavior of Na2SO4- LDT composite solid electrolyte. J Adv Res 7:135–141
Chandra A (2013) Synthesis and dielectric studies of PEO-PVP blended solid polymer electrolytes. Pure Appl Phys 51:788–791
Ben Gzaiel M, Oueslati A, Hlel F, Gargouri M (2016) Synthesis, crystal structure, phase transition and electrical conduction mechanism of the new [(C3H7)4N]2MnCl4 compound. Phys E Low-dimensional Syst Nanostructures 85:405–413
Rahmouni H, Smarib M, Cherifa B, Dhahrib E, Khirounia K (2015) Conduction mechanism, impedance spectroscopic investigation and dielectric behavior of La0.5Ca0.5-xAgxMnO3 manganites with the composition below the concentration limit of silver solubility in perovskites (0≤x≤0.2). Dalt Trans 44:10457–10466
Ajili O, Louati B, Guidara K (2018) Electrical properties and conduction mechanism by CBH model of Na2SrP2O7. J Mater Sci Mater Electron 29:8649–8659
Chakchouk N, Louati B, Guidara K (2018) Electrical properties and conduction mechanism study by OLPT model of NaZnPO4 compound. Mater Res Bull 99:52–60
Nasri S, Megdiche M, Gargouri M (2014) Electrical conduction and dielectric properties of a newly synthesized single phase:Ag0.4- Na0.6FeP2O7. Phys B Phys Condens Matter 451:120–127
Kufian MZ, Majid SR, Arof AK (2007) Dielectric and conduction mechanism studies of PVA- orthophosphoric acid polymer electrolyte. Ionics (Kiel) 13:231–234
Acknowledgements
The authors would like to thank the Ministry of Education for Fundamental Research Grant Scheme (FRGS/1/2018/STG07/UNIKL/02/8) and Universitas Islam Riau- Universiti Teknologi PETRONAS for grant scheme (Grant No: 015ME0-041).ssssss
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohamed, A.S., Shukur, M.F., Kadir, M.F.Z. et al. Ion conduction in chitosan-starch blend based polymer electrolyte with ammonium thiocyanate as charge provider. J Polym Res 27, 149 (2020). https://doi.org/10.1007/s10965-020-02084-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-020-02084-7