Abstract
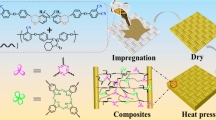
A low-temperature curable phenolic/benzoxazine-functionalized phthalonitrile (SH/BZ-CN) copolymer system with well processability is designed and applied in high performance glass fiber (GF) composite laminates. Differential scanning calorimetry (DSC) results showed that plenty of phenolic hydroxyl groups on SH could catalyze the oxazine ring-opening and triazine/phthalonitrile ring-forming reaction of BZ-CN. The ring-opening peak and ring-forming peak of SH/BZ-CN systems are reduced by 47.1 °C and 17.0 °C than those of BZ-CN, respectively. The processability of SH/BZ-CN copolymers were improved and could be controlled by tuning SH content, processing temperature and time. These parameters provided ground for preparing SH/BZ-CN/GF composite laminates under a relatively mild condition. All SH/BZ-CN/GF composite laminates exhibit excellent flexural strength more than 500 MPa and flexural modulus over 22.0 Gpa. SH/BZ-CN/GF composites showed immiscible structures and double Tgs, and they could stand high temperature up to 350 °C. Low temperature curing, short processing time and low processing pressure are beneficial to large-scale manufacturing and application of SH/BZ-CN/GF composites.









Similar content being viewed by others
References
Laskoski M, Dominguez DD, Keller TM (2013) Alkyne-containing phthalonitrile resins: controlling mechanical properties by selective curing. J Polym Sci A Polym Chem 51(22):4774–4778
Keller TM (1988) Phthalonitrile-based high temperature resin. J Polym Sci A Polym Chem 26(12):3199–3212
Zhang J, Liu X, Wen H, Xie M, Cai X (1997) Investigation of the properties of phthalocyanine resin containing bismaleimide groups. Polym Int 42(4):363–366
Zuo F, Liu X (2010) Synthesis and curing behavior of a novel benzoxazine-based bisphthalonitrile monomer. J Appl Polym Sci 117(3):1469–1475
Sastri SB, Armistead JP, Keller TM (1996) Phthalonitrile-carbon fiber composites. Polym Compos 17(6):816–822
Keller TM, Price TR (1982) Amine-cured bisphenol-linked phthalonitrile resins. J Macromol Sci Chem 18(6):931–937
Dominguez DD, Keller TM (2006) Low-melting phthalonitrile oligomers: preparation, polymerization and polymer properties. High Perform Polym 18(3):283–304
Zeng K, Yang G (2012) Phthalonitrile matrix resins and composites. John Wiley & Sons, Inc., New Jersey
Keller TM (1994) Synthesis and polymerization of multiple aromatic ether phthalonitriles. Chem Mater 6(3):302–305
Sastri SB, Keller TM (1999) Phthalonitrile polymers: cure behavior and properties. J Polym Sci A Polym Chem 37(13):2105–2111
Sumner MJ, Sankarapandian M, McGrath JE, Riffle JS, Sorathia U (2002) Flame retardant novolac–bisphthalonitrile structural thermosets. Polymer 43(19):5069–5076
Keller TM (1992) Strong organic acid cured phthalonitrile resins for high temperature applications. Polym Prepr (USA) 33(1):422–423
Burchill PJ (1994) On the formation and properties of a high-temperature resin from a bisphthalonitrile. J Polym Sci A Polym Chem 32(1):1–8
Zeng K, Zhou K, Zhou S, Hong H, Zhou H, Wang Y, Yang G (2009) Studies on self-promoted cure behaviors of hydroxy-containing phthalonitrile model compounds. Eur Polym J 45(4):1328–1335
Zhou H, Badashah A, Luo Z, Liu F, Zhao T (2011) Preparation and property comparison of ortho, meta, and para autocatalytic phthalonitrile compounds with amino group. Polym Adv Technol 22(10):1459–1465
Duro JA, de la Torre G, Barberá J, Serrano JL, Torres T (1996) Synthesis and liquid-crystal behavior of metal-free and metal-containing phthalocyanines substituted with long-chain amide groups. Chem Mater 8(5):1061–1066
Augustine D, Mathew D, Nair CR (2015) Phthalonitrile resin bearing cyanate ester groups: synthesis and characterization. RSC Adv 5(111):91254–91261
Zou X, Xu M, Jia K, Liu X (2014) Synthesis, polymerization, and properties of the allyl-functional phthalonitrile. J Appl Polym Sci 131(23)
Chaisuwan T, Ishida H (2006) High-performance maleimide and nitrile-functionalized benzoxazines with good processibility for advanced composites applications. J Appl Polym Sci 101(1):548–558
Zhang B, Luo Z, Zhou H, Liu F, Yu R, Pan Y, Zhao T (2012) Addition-curable phthalonitrile-functionalized novolac resin. High Perform Polym 24(5):398–404
Keller TM (1993) Imide-containing phthalonitrile resin. Polymer 34(5):952–955
Xu M, Yang X, Zhao R, Liu X (2013) Copolymerizing behavior and processability of benzoxazine/epoxy systems and their applications for glass fiber composite laminates. J Appl Polym Sci 128(2):1176–1184
Dominguez DD, Keller TM (2008) Phthalonitrile-epoxy blends: cure behavior and copolymer properties. J Appl Polym Sci 110(4):2504–2515
Guo H, Lei Y, Zhao X, Yang X, Zhao R, Liu X (2012) Curing behaviors and properties of novolac/bisphthalonitrile blends. J Appl Polym Sci 125(1):649–656
Zou X, Xu M, Jia K, Liu X (2015) Copolymerizing behavior and processability of allyl-functional bisphthalonitrile/bismaleimide system. Polym Compos 38(8):1591–1599
Ghosh NN, Kiskan B, Yagci Y (2007) Polybenzoxazines—new high performance thermosetting resins: synthesis and properties. Prog Polym Sci 32(11):1344–1391
Yagci Y, Kiskan B, Ghosh NN (2009) Recent advancement on polybenzoxazine—a newly developed high performance thermoset. J Polym Sci A Polym Chem 47(21):5565–5576
Agag T, Takeichi T (2003) Synthesis and characterization of novel benzoxazine monomers containing allyl groups and their high performance thermosets. Macromolecules 36(16):6010–6017
Kiskan B, Colak D, Muftuoglu AE, Cianga I, Yagci Y (2005) Synthesis and characterization of thermally curable benzoxazine-functionalized polystyrene macromonomers. Macromol Rapid Commun 26(10):819–824
Liu YL, Yu JM (2006) Cocuring behaviors of benzoxazine and maleimide derivatives and the thermal properties of the cured products. J Polym Sci A Polym Chem 44(6):1890–1899
Kumar KS, Nair CR, Ninan KN (2009) Investigations on the cure chemistry and polymer properties of benzoxazine–cyanate ester blends. Eur Polym J 45(2):494–502
Kimura H, Ohtsuka K, Matsumoto A (2011) Curing reaction of bisphenol-A based benzoxazine with cyanate ester resin and the properties of the cured thermosetting resin. Express Polym Lett 5(12):1113–1122
Li X, Gu Y (2011) The co-curing process of a benzoxazine-cyanate system and the thermal properties of the copolymers. Polym Chem 2(12):2778–2781
Lin CH, Huang SJ, Wang PJ, Lin HT, Dai SA (2012) Miscibility, microstructure, and thermal and dielectric properties of reactive blends of dicyanate ester and diamine-based benzoxazine. Macromolecules 45(18):7461–7466
Wang MW, Jeng RJ, Lin CH (2015) Origin of the rapid trimerization of cyanate ester in a benzoxazine/cyanate ester blend. Macromolecules 48(8):2417–2421
Liu J, Scott C, Winroth S, Maia J, Ishida H (2015) Copolymers based on telechelic benzoxazine with a reactive main-chain and anhydride: monomer and polymer synthesis, and thermal and mechanical properties of carbon fiber composites. RSC Adv 5(22):16785–16791
Kornmann X, Berglund LA, Lindberg H (2000) Stiffness improvements and molecular mobility in epoxy-clay nanocomposites MRS Online Proceedings Library Archive, 628
Acknowledgements
The authors wish to thank for financial support of this work from the South Wisdom Valley Innovative Research Team Program and Guangdong Shunde Great New Materials Co., Ltd..
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yang, X., Li, K., Xu, M. et al. Designing a low-temperature curable phenolic/benzoxazine-functionalized phthalonitrile copolymers for high performance composite laminates. J Polym Res 24, 195 (2017). https://doi.org/10.1007/s10965-017-1360-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-017-1360-y