Abstract
Nanogels have become an important topic of interdisciplinary research, especially in the fields of polymer chemistry, physics, materials science, pharmacy, and medicine where their small dimensions prove highly advantageous. One of the most important areas of research and development concerning these gels is in drug delivery applications. Nanogels could potentially revolutionize conventional therapy and diagnostic methods because of their superior effectiveness over their macro-sized counterparts in almost all therapeutic areas. Current strong interests in this class of material have driven many studies to discover novel production methods and new areas of application in this area. Therefore, it is important to keep abreast of the development of these gels. In this review, we aim to cover the basic aspects of organic nanogels including their definition, classification, and synthesis methods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polymeric submicron gels (nanogels and microgels) were once regarded as unwanted by-products of the polymerization process. These gels have now become increasingly important in many areas of research due to their diverse and useful properties. These properties include a high loading capacity for therapeutics such as drugs [1], imaging agents, proteins [2], DNA [3], and RNA [4]. These gels also have space for ligand attachments, high mechanical strength [5] that affords a higher resistance to degradation, and the ability to prolong the circulation period of cargo loading in the blood stream [6,7,8]. The gels’ small size and thus enhanced permeability [9] allows for efficient delivery of drugs to tumor sites and enables them to act as a protective layer for payloads [10,11,12]. These minute gels are now being widely used in many fields that include chemistry, physics, medical, pharmaceutical, and materials science. Such strong interests in this class of material have been the main drive for many studies to discover novel production methods and new areas of application in this area. Therefore, it is important to keep abreast of the development of these gels. In this review, we aim to cover the basic aspects of organic nanogels including their definition, classification, and synthesis methods.
Definition of submicron gels
In general, a gel is defined as a continuous colloid or polymeric network that fills an entire volume. A gel may be formed by covalent cross-linkages, physical interactions among its chains that could form aggregation, formation of glassy junctions by co-polymers, interlamellar interaction of polymers, and precipitation of flocculents in a polymeric network with a large structural deviation [13]. Submicron gels differ from the general description of the gel above, as they are a finite colloidal or polymeric network of which the pores are fully or semi-permeable to molecules of solvents with dimensions ranging from nano to micrometers. IUPAC in its “Gold Book” gave a straightforward definition of these gels according to size, wherein the size of the nano- and microgels were given as 1–100 nm and 0.1–100 μm, respectively [13]. The book also states that both nano and microgels could be gel particles of any shape from the above-mentioned size ranges. An alternative to this size-based definition can be found in [14], i.e. nanomaterials in general can be defined according to its applications—for biomedical applications, structures of up to 1000 nm may still be considered as nanostructured materials. Another definition of submicron gels is based on the occurrences of internal cross-linkages that covalently bind all the side chains in a single molecule [15]. This definition enables submicron gels to be classified as a new class of materials along with other types of macromolecules such as linear, branched, dendrimers, etc.
Classification of submicron gels
Gels, regardless of size and shape, natural or synthetic, can always be divided into two main groups. The first group consists of physical gels or pseudogels. This class of gel is formed via relatively weak physical bonds [16,17,18,19,20] such as Van der Waals, hydrogen bonding, hydrophilic/hydrophobic, or electrostatic interactions. Usually, gels in this group are formed via the self-assembly action of block co-polymers and/or graft co-polymers, which translates to weak binding forces. For example, increasing the applied stress, temperature, or even changing the pH or solvent would easily affect the physical structure of these gels.
The second group of gel consists of permanent gels. This type of gel is formed via relatively strong covalent bonds [21,22,23,24] that hold the shape of the gels. These permanent bonds give the gels the ability to sustain their shape under stress up until the degradation of the whole structure. This review will only focus on this second group of gels.
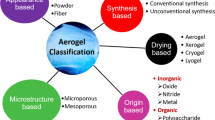
Generally, all types of submicron gels are referred to as submicron particles. A more specific classification apart from size range is therefore vital. Further details such as the morphology (capsules, sphere, rod, etc.), homogeneity/heterogeneity of the building monomer/polymer (polymer conjugates, etc.), and structure (layer-by-layer, core-shell particles) of the gels can also be taken into account. Depending on the factors mentioned above, submicron gels could be divided into classes, as show in Fig. 1.
Some examples of submicron gels are discussed in the following sections. The plain nanosphere is the most basic form of submicron gels. It is made up of a solid core of polymeric chains. The inner core can either be in the form of linear polymeric chains in a coiled position or a crosslinked polymeric network.
A nanocapsule resembles a submicron gel system with a thin polymeric skin encapsulating a reservoir or cargo. The outer layer of the nanocapsule consists of polymeric chains that are linked by physical forces or even chemical cross-linkages.
Hybrid submicron gels are based on a combination of polymeric gels and inorganic material. An example can found in the work of Zhu et al. [25] (Fig. 2). This type of gel has many advantages including stimuli-responsive properties based on volume phase transition.
An example of hybrid nanogels from Bi2O3. This combination provides the PVA with a heat stimuli characteristic. Reproduced from [25]
Biohybrid nanogels are gels that consist of synthetic polymers and biomolecules such as microorganisms, enzymes, peptides, and biopolymers [26] as their building blocks. The synthetic part in hybrid molecules contributes towards their overall mechanical properties, stability in aqueous phase, and sites for incorporation of reactive groups for conjugation with dyes or other specific markers. On the other hand, the content of the biomolecules reflects their biological mechanism. These types of nanogels have unique properties and huge potential in terms of application that are not seen in other types of nanogels.
Materials variety
Submicron gels are considered engineering materials, and are thus expected to be superior over their macro-sized counterparts in terms of properties. They are designed to enhance the effectiveness of bioactive substances, therapeutic agents, imaging substances, etc., by improving their solubility, size, biodistribution, release kinetic, delivery, stability, and biocompatibility, among others, as well as providing a special passage across biological barriers. These properties are achieved by the introduction of a variety of materials according to the desired effects of the gels.
Drug solubilizing properties can be enhanced by altering the hydrophilicity or hydrophobicity ofsubmicron gels core material, depending on the inherent solubility of the drug itself. Theselection of certain molecular weight values enables the control of the size distribution of the gels, while drug loading and release properties can be carried out by material selection and/or the adjustment of crosslinking density. In the case of triggered-release delivery, submicron gels thatare sensitive towards stimuli such as temperature and pH can be designed by the incorporation ofmaterials such as PNIPAAM and PAA respectively. The introduction of hydrophilic materialsinto the building blocks of submicron gels may prolong circulation in blood, since hydrophilicmaterials such as PEG are known to cause a steric effect that prevents serum opsonins actionfrom reticuloendothelial system (RES). PEG also provides stealth properties for enhancedbiocompatibility[27]. Biodegradability properties, on the other hand, can be exerted by theincorporation of either synthetic or natural based polymers[28]. Lorenzo et al. reported thatnanogels with tailorable phase transition behavior can be obtained by controlling the amount ofits constituting block copolymers of various phase transition behaviors [29]. Kumar et al.demonstrated that the use of 4000 g/mol PEGDMA in PEGDMA/MAA microgels resulted in thelargest microgels with the highest insulin loading efficiency [30]. On the other hand, the samematerial used for the same nanogels may yield a significant difference in terms of result usingdifferent synthesis methods [31]. Table 1
lists the typical materials used to build submicron gels.
Applications of submicron gels
Submicron gels have wide-ranging applications in medical and industrial fields. In medical applications, they are designed according to intended therapeutic categories, target areas and administration modes. Design consideration includes various moieties that enabled attachment or conjugation of ligands for diagnostic, biomarker, molecular imaging, gene delivery, targeted delivery or drug. Like-wise, in industrial applications, they are usually used as support materials for synthesis of active inorganic materials that can be used as catalysts. Industrial applications include water purification i.e. arsenic removal from drinking water [32], oil spill recovery [33], ultra-hydrophobic coating [34]. Examples of submicron gels applications can be found in Table 2.
Size effect on different therapeutic areas
Important characteristics of nano/microgels include size, shape and surface properties. These factors are crucial for their in vivo biodistribution, since different therapeutic areas involve different barriers that present specific requirements during delivery. Size has a significant influence on gels circulation time in blood, biodistribution, tissue penetration and the fate of therapeutic agents. The widely accepted ideal size for nano/microgels in delivery applications is 70-200 nm. Particles larger than a micron will be opsonized, and results in accumulation in the liver and spleen, leading to a higher risk of aggregation. On the other hand, gel particles less than 5 nm are cleared rapidly by way of renal clearance and extravasation [79, 80]. Surface properties such as charge also have effect on renal clearance, where particles of the same size but with negative charge are not filtered through the glomerular basement membrane [81]. Additionally, a low positive charge value on particles may enhance cellular uptake, while at a higher value, toxicity issues may surface.
Therapeutic areas determine a certain practical size for active compound delivery. For example; lipophilic molecules (<600 Da) can penetrate into the skin through transdermal passive delivery [82]. Transdermal protein or peptide delivery such as insulin can be done using highly flexible vesicles such as transfersomes. Under non-occulated conditions and with hydration gradient, transfersomes, with a size of up to 200-300 nm can squeeze through skin pores that are one-tenth of the vesicles.
Tumors replicate faster compared to healthy tissues, and nutrients from the bloodstream are dominated by them as a result – potentially reducing healthy tissues to an inactive state. The rapid development of the tumor tissue hinders the nutrient supply to the inner layer of the tissue, leading to a build-up of necrotic core [83]. In order to continue growing beyond this point, the tumor stimulates rapid but poorly organized neovascularization [84] for nutrient supply. The newly-formed heterogenous blood vessels have high vascular permeability that allows the penetration of particles less than 100 nm through extravasation. On the other hand, a tumor also has poor lymphatic drainage which allows for the accumulation of anticancer drugs. The enhanced permeability and retention effect (EPR) of the tumor tissue enables nano/microparticles to be used as targeted drug delivery agents. However, therapy of the brain tumor is more complex due to the blood-brain barrier. The blood-brain barrier is formed by a tight endothelial layer that prevents passive accumulations of molecules in the brain. The pore size of the affected blood-brain barrier is around 12 nm, while it is smaller for the healthy tissue [8]. Particles 30-600 nm in size were tested with successes in animals, but only with the use of BBB permeating ligands [84].
Synthesis of submicron gels
Numerous methods to produce submicron gels have been presented in the literature. These gels can be produced via direct polymerization in homogenous or heterogeneous mixtures of different types of monomers, self-assembly mixtures, in an emulsion system, or in template-based nanofabrication such as Particle Replication in Nonwetting Templates (PRINT) or even polymerization involving systems with larger polymeric structures as their building blocks.
Normally, all types of production methods involve either grafting and/or crosslinking polymerization to form covalent bonds in the polymeric molecule backbone. In such processes, the substrate—be it a monomer or polymer—must contain at least one functional group that is polymerizable by the actions of radicals generated in the system.
Basically, radicals may be generated by either dissociation of ammonium persulfate in water [35, 85-87], degradation of chemical initiators such as N,N′-methylene-bis-acrylamide (classical chemistry) upon absorption of UV light photo initiator [87, 88] or radiolysis of water via ionizing radiation [78, 89, 90].
In the production of submicron gels, the polymerization process may take place in a solution (dilute solution polymerization) or sometimes involve the mechanism of emulsification (emulsion-based polymerization) to restrict the size of the final products as well as to avoid aggregation. Emulsification can be achieved using a certain type of surfactant depending on the design of the emulsion system.
Emulsion-based crosslinking polymerization
The synthesis of covalent-bonded submicron gels usually involves polymerization in a solution environment. In this case, the most important aspect of this process is to keep the polymerization in a confined space of which the size would depend on the size of the intended final product. Such confined spaces, which are also commonly known as micro or nanoreactors, are often formed using oil-in-water (direct) or water-in-oil (inverse) micelles.
Micelles—formed from the arrangement of surfactant in oil/water interphase—constitute micro or nanoreactors that are separated from each other [91]. Polymerization and crosslinking reactions in micro or nanoreactors normally leads to high monomer conversion [92, 93], making emulsion-based crosslinking polymerization a preferred technique in submicron gel production [94].
In a typical emulsion-based polymerization reaction (oil-in-water), radicals are generated from decomposition of the chemical initiators—also known as water radiolysis. These chemical initiators are normally generated outside of the micelles, where they react with monomers. The reacted monomers then transform into monomer radicals, and enter the emulsion via diffusion. The diffused monomer radicals would then react with other unreacted monomers. This will result in chain growth [15].
Emulsion-based polymerization may occur in both microemulsion and nanoemulsion. Although both types of emulsion are essentially prepared using the same materials (i.e. oil, water, and surfactant), the difference between the two lies in the thermodynamic stability [95] of both types of emulsions, which may render different effects on the final polymerization product. Further details regarding this issue will not be discussed in this review.
Direct micelles polymerization technique
Typically, emulsion polymerization processes involve several steps such as preparation of multilayer emulsion, and repeated dispersion or precipitation in certain sequences. These processes can sometimes be temperature and/or pH sensitive during synthesis. Unless justified, these techniques are normally avoided, as they are time consuming and expensive. Normally, a much simpler process, where a facile one-step polymerization reaction can be achieved, is always preferable.
Landfester et al. [34] studied varieties of miniemulsion techniques to synthesize polydimethylsiloxane (PDMS) nanogels. In their study, PDMS nanogels with sizes ranging from 40 to 1100 nm were obtained. They reported that the confinement provided by the small emulsions enabled more length scale copolymerization, thus highly crosslinked particles were obtained. The direct emulsion polymerization technique can also be used to synthesize magnetic-responsive nanogels from 4-vinylpyridine P (4-VP) [32]. Deen et al. [36] synthesized and characterized Poly (N-isopropylacrylamide) nanogels. They conducted an emulsion polymerization above the LCST and used a systematic influence of addition of surfactant on the final size and structure of the nanogels. Polymerization can also be initiated from the decomposition of chemical initiators using an argon laser exposure. In one study, amphiphilic nanoparticles were synthesized with a photo polymerization method [37]. In this study, direct micelles emulsion consisting of monomers, triethanolamine, and eosin Y were exposed to argon laser with a wavelength range of 480 to 520 nm for 1 h at room temperature. The DLS measurements showed that the nanoparticles produced ranged from 10 to 20 nm in size. Another study investigated the synthesis of dual-responsive nanogels using a novel initiator made from PEGylated AIBN [38]. The PEGylated AIBN was first synthesized using the method described in Walz et al. [96]. The product, PEG-AIBN-PEG, was then introduced to improve the macro-state and stability of the nanogels produced using this method.
Sometimes, direct micelles are preferred over inverse micelles for polymerization purposes due to the sensitivity of monomers towards water hydrolysis. One study synthesized nanogels from Poly(N-vinylcaprolactam) via polymerization using the direct micelles method [39]. First, the PVCL monomers, toluene, hexadecane, and AIBN were mixed with SDS in water. Polymerization was then carried out at 72 °C in 500-rpm stirring conditions, for 17 h. The study reports that the problems of monomer hydrolysis, low-solid content of the dispersions, and the nanogel re-dispersion in water were eliminated using this method. Natural-based submicron gels from deoxycholate (DE) and carboxymethyl (CM) chitosan were formed via the chemical crosslinking of emulsified chitosan solution in methylene dichloride. The final product was then studied using TEM, the size of which was determined to be 200–600 nm [97].
Direct micelles polymerization may also offer a clean technique for producing nanogels, whereby polymerization can be done in an aqueous medium with zero or small amounts of surfactant. An example of this method can be found in the synthesis of PVCL-based nanogels with ketal linkages [40] and poly-(oligo(ethylene glycol) methacrylate) (POEGMA)-based nanogels with tunable thermosensitivity properties [42].
Emulsion polymerization can also be combined with other techniques to achieve metabolic thiol-cleavable submicron gels. Nanogels from 6-O-vinyladipoyl-D-galactose (ODGal) were first synthesized via the enzymatic transesterification technique. These nanogels were then crosslinked with VCL and MAA via free radical emulsion polymerization [43]. More recent examples can be found in Sengel et al. [44] and Yi et al. [45]. Table 3
provides concise information pertaining to the selected methods discussed under this subsection. A typical polymerization process in direct micelles system is elucidated in Fig. 3
.
Inverse micelles polymerization technique
Normally, emulsion-based polymerization involves polymerization reactions of polymers in an aqueous matrix. This suits hydrophobic polymers well because in normal emulsion the surfactants that constitute micelles are arranged in such a way that the hydrophobic part of the surfactants are pointed inwards, away from the aqueous matrix. In this way, the confined spaces in the micelles would be hydrophobic and thus be more suitable for containing hydrophobic polymers. In cases where hydrophilic polymers are the reactants, the hydrophilic spaces to contain these polymers can then be achieved by inversing the arrangement of the surfactants. These types of micelles are known as inverse micelles (water-in-oil) (Fig. 4).
Inverse micelles polymerization has been proven to yield stable and uniform micron-sized polyacrylamide latexes of high molecular weight [98]. Candau et al. demonstrated that nanoparticles from a polyacrylamide latex can be synthesized using either exposure to thermal condition at 45 °C or UV irradiation at 25°C [33]. In both cases, free radicals were generated by decomposition of azobisisobutyronitrile (AIBN). It was reported that the monomer, acrylamide, acted as a co-surfactant that contributed to the stability of the inverse micelles. Additionally, the viscosity of the final product decreased due to a reduction in particle interactions.
Although PVCL is prone to water hydrolysis, being a hydrophilic polymer, the inverse micelle system is usually preferred for polymerization. In one study, biocompatible and thermally sensitive poly(N-vinylcaprolactam) nanogels were synthesized using an inverse microemulsion polymerization technique [41]. Natural-based polymers can also be polymerized in inverse micelle spaces to produce submicron gels. One example is the modification of hyaluronan acid (HA) [46]. HA microgels can also be obtained via attachment of aldehyde and hydrazide functionalities through oxidation with sodium periodate and coupling with glutahydrazide, respectively, in inverse micelles [70]. Another example of natural-based nanogels produced using the inverse micelles polymerization technique is a crosslinked star shaped acrylate arms with amine groups from various amino acids of a hen egg’s ovalbumin [47]. Another study also investigated the microspheres from chitosan that were produced by the introduction of covalent bonds among its amino group with the use of different crosslinking agents such as glutaraldehyde, sulphuric acid, and heat treatment [48].
One study used the above-mentioned method to synthesize nanogels with long-term stability and super low fouling ability against nonspecific protein adsorption from blood using poly(N-2-hydroxyethyl acrylamide) (polyHEAA) and acrylic acid (AA) [69]. As described in this work, the oil phase was prepared by mixing hexane, tween 80, Span 80, and ‘2,2’-azobis’(4-methoxy-2,4-dimethyl valeronitrile) (V-70), after which this mixture was kept on ice. The aqueous phase was prepared by dissolving HEAA, AA, and MBAA in 1 ml of DI water. Both the aqueous and oil phases were then added to a container and were shaken vigorously and sonocated for 2 min; the mixture was then purged with nitrogen. Polymerization was carried out at 40 °C for 4 h under nitrogen purging.
Inverse micelles polymerization is usually preferable against direct micelles polymerization, especially in the synthesis of submicron gels from polymers with good water solubility in both its monomer and polymer forms. In the case of polyacrylic acid (PAA), where both AA and PAA are soluble in the same polar medium during processing, formation of its submicron gels is impossible to control. Therefore, an inverse micelle system is a good alternative [49]. More recent works concerning this type of synthesis are described in Sahiner et al. [51], Su et al. [52], Hsiao et al. [53], Sun et al. [54] and Zhang et al. [55]. Table 4
provides a summary of the selected methods discussed under this subsection.
Surfactant-free micelles polymerization technique
Emulsion polymerization (Fig. 3) may come with several advantages, as discussed in the preceding section, but nevertheless, a few issues associated with utilization of surfactant are its main disadvantage. Surfactant and co-surfactant may sometimes become entrapped in the final product, thus it could be impossible to purify the final product from the bound surfactant molecules. However, as previously mentioned, emulsion-based polymerization can also be done in a surfactant-free system.
Surfactant-free emulsion polymerization has a few advantages in terms of submicron gel preparation. Clean and applicable surfaces can be readily obtained via this method [74]. Some examples can be found in a report by Singka et al. where pNIPAM nanogels were formed by grafting butyl acrylate (BA) [75] onto pNIPAM backbone and Zhou et al. where a POEGMA microgel was synthesized via the emulsifier free radical polymerization Fig. 5
technique [57].
Submicron gels can also be prepared using a chemical process that is known as heterogeneous free radical polymerization. This method normally involves monomers, chemical stabilizers, and chemical initiators that are soluble in its corresponding solvents. As the polymerization initializes, the resulting polymers will become insoluble in the solvent. As the polymers precipitate, the chemical stabilizers will stabilize the polymers by forming well-dispersed microgels. Some examples of this method can be seen in the preparation of PS [75], poly(methyl methacrylate) (PMMA) [76], and Poly(HFMA-co-NIPAM) [58] microgels.
Additionally, in one study, hydrophilic nanogels from NIPAM and VP were copolymerized in solution form without the use of a surfactant [59]. In another, hybrid nanogels containing lanthanum fluoride and copolymers were synthesized using the method of precipitation polymerization without surfactant [71]. The absence of surfactant in emulsion polymerization may cause emulsion instability, however, this issue can be overcome with the use of comonomers, as shown in a few researches [99, 100]. Comonomers can often act as a surfactant, as it normally has its own hydrophilic and hydrophobic parts. Hydrophilic comonomers normally surround the surface of the hydrophilic monomer, thus providing stability to the original emulsion.
Karabacak et al. [74] studied the effect of different types of monomers and pH value in the surfactant-free polymerization of PS colloids. In this study, 2-(dimethylamino)ethylmethacrylate (DMA) and methyl methacrylate (MMA) were found to be effective comonomers in a PS nanoparticle preparation via surfactant-free polymerization at different pH. The more hydrophilic the comonomers used in the process, the smaller the final particles produced.
A particular method of synthesis has been gaining popularity recently due to its minimalistic procedures. Known as the one-pot synthesis technique, this method has the advantage of simplicity. Additionally, the final product size can be easily tuned simply by varying the reactant concentration. In order to further overcome the need to use surfactants and any kind of stabilizer, Tang et al. [72] suggested a facile one-pot method in the preparation of nanogels through the combination of a step-growth polymerization with thermally-induced phase separation. The nanogel formation was initiated through the phase separation of thermo-sensitive polymers, where precursor particles formed. This was followed by aggregation and subsequent polymerization crosslinking of the precursor particles through an amine-epoxide reaction. Poly(propylene oxide) (PPO)-containing tri-amine (trade name Jeffamine T-403) and 1,3- butadiene bisepoxide were mixed at an amine to epoxide ratio of 1:0.95. The mixture was then incubated at 65 °C in a water bath for 15 min and rapidly cooled down to room temperature and diluted. The process was then repeated to grow the nanogels. The study reported that a higher monomer concentration would result in larger particles.
The one-pot facile method was used to synthesize nanogels with glucose-sensitive function [60]. Ramos et al. synthesized core-shell hybrid nanogels from N-vinylcaprolactam (VCL) with a 3-(trimethoxysilyl) propylmethacrylate (TPM) silica-based core [61]. Another study used the same method to synthesize HPMC/PAA hybrid nanogels in an aqueous-phase non-surfactant setup [62].
One study describes the synthesis of bioreducible and acid labile nanogels/microgels synthesis as “very simple” [101]. In the study, the nanogels were synthesized using this method without use of surfactants, stabilizers, or additives. However, the precursor materials were constructed using several pre-synthesis steps that in of itself had a yield value of 58–79%. pH-responsive Poly(vinyl-amine) (PVAM) microgels were synthesized in one other study using a two-step method based on a non-aqueous dispersion (NAD) polymerization method [63]. Nano or microgels can be used as a building block to form a nano-product. As an example, several researchers investigated the preparation of silver nanoparticles using an in situ reduction method of Ag + ions in NIPAM microgels [102]. Additionally, surfactant and organic solvents can both be avoided in this context. In a recent study, a pH-responsive alginate nanogel was synthesized using the one-pot technique without surfactant and organic solvent [103]. Recent works under this category can be found in Zhao et al.[64] and Abreu et al.[65]. Summary of selected methods under this subsection is given in Table 5.
Lithographic method
The advancement of submicron gels application in the field of modern diagnostics, advanced catalytic, treatment, etc. has initiated the development of vastly complex particles. These applications place great emphasis on the functionality of the candidate materials. On the other hand, hydrogels, in general, offer a great template for the development of designer chemicals such as smart structures that are able to react to stimuli such as pH, heat, electric potential, photon, etc. A technique known as lithography offers a high degree of control in engineering gels. Some examples of materials development using this method are briefly discussed in the following section.
One study involved the synthesis of a PEG micro ring using a soft lithographic method [73]. Briefly, PDMS was stamped with confined spaces in the order of picoliter and then put in contact with a flat PDMS film stained with a hexadexanethiol (HDT) ethanol solution. Next, a drop of PEGDA mixture with a photoinitator was applied to the stamp. The stamp was then pressed on a glass slide at 30 °C for 4 h followed by cooling to 4 °C. The PDMS stamp was lifted and the patterned substrate was annealed. The crosslinking process was initiated at 10 °C for 10 min using UV photo irradiation. A method that was developed based on the lithographic technique known, as Particle Replication in Nonwetting Templates (PRINT), as described in [66], was used to synthesize PEG microsized gels with variable sizes, shapes, and compositions.
One of the most recently explored areas in lithographic methodologies is the microfluidic technique. This technique affords a high degree of control in the design of intended colloids and narrow-size distributions. In this technique, a liquid monomer and its immiscible solvent reservoirs are channeled at an elevated pressure through specialized fabricated devices that consist of tapered micro channels with junctions, where the two liquids merge forming an emulsion and micro droplets. The crosslinking process can be done either by UV irradiation or polycondensation [67, 104].
Microgels with a pH-responsive character was developed using a microfluidic technique [68] for cell encapsulation and programmed release. Fabrication was done in 2 steps. First, cytocompatible dendritic polyglycerols (dPG) with acid-cleavable linkers were prepared. These macromonomers were then channeled through a microfluidic device together with a fibroblast cell (NIH3T3) from a mouse and homo-bifunctional PEG-dicylooctyne as the crosslinker. The crosslinking process in the emulsified mixture was then continued through bio-orthogonal strain-promoted azide–alkyne cycloaddition (SPAAC). The degradability of the final microgels could then be altered using dPG with different acid-cleavable linkers that would degrade with different kinetic conditions depending on the pH of the environment.
The microfluidic technique—while offering a high degree of control in the architecture of colloids and a narrow-sized distribution, as well as allowing for a surfactant and organic solvent-free process—also comes with a few limitations. If this method is to be employed for encapsulation of bioactive materials, the design of the whole procedure must be set so that the bioactive materials would not interact with the monomer, solvent, and other additives. Furthermore, this approach depends heavily on a high level of precision that is required in the fabrication of micro-moldings and other design aspects. Table 6
gives a summary on size and parameters on methods discussed under this subsection.
Irradiation method
Staudinger et al. first reported the application of an irradiation-induced method in submicron gels in the 1930s, where styrene divinylbenzene microgels were formed upon exposure to irradiation. Subsequently, more and more works have since been reported, covering a multitude of different methods and microgels from different starting materials. The irradiation-induced method has since become more popular due to its additive-free initiation, ability of crosslinking/grafting, particle size control, and its functionalization and sterilization in synthesizing submicron gels, which can be achieved in just one step.
Generally, radiation-induced synthesis of submicron gels can be classified into two groups, and this depends on the selection of the reactant that will influence the dominant crosslinking process that ensues during the recombination of radicals. The first group is characterized by inter-molecular crosslinking, often involving reactants from the monomer or its mixtures. The second group emphasizes on the intra-molecular crosslinking of one single polymer [15, 105].
Submicron gels are formed when a monomeric/polymeric solution or emulsion is exposed to ionizing irradiation. The radiation energy is mostly absorbed by the water component up to a certain energy threshold; at which point the water molecules will decompose into several short-lived radicals. Among these, the hydroxyl radicals and hydrogen atoms will be the two radicals with the potential to convert a monomer or polymer into microradicals i.e. via hydrogen abstraction. The recombination of two separate microradicals will lead to intermolecular crosslinking, normally accompanied with an increase in molecular weight and the eventual formation of a large single macroscopic gel due to an exceedingly high degree of crosslinking. On the other hand, recombination of radicals from the same chain is known as intra-molecular cross-linking, where separate micromolecules in nano and micro scales can be obtained. Recombination of radicals under this condition does not affect the molecular weight of the resulting gel. Several examples of ionizing radiation-induced syntheses of submicron gels are discussed in the following section.
Ulanski et al. synthesized PAA nanogels by irradiating PAA at a pH of 2.0 in a closed-loop system with continuous flow of PAA. They reported that at a higher pH, chain scission is more likely to occur due to the repulsive forces between negatively charged polyelectrolyte chains. In order to promote crosslinking, the pH of the polyelectrolyte solution was adjusted to 2.0 to neutralize the repulsive forces that prevent adjacent polymers to merge. Furthermore, to maximize intramolecular crosslinking, the synthesis was done by irradiating a dilute solution of PAA with a high dose rate of electron beams in pulse mode. The average molecular weight, intrinsic viscosities, and radius of gyration data suggest that intramolecular crosslinking has taken place and nanogels have been formed [50]. The same group of researchers also synthesized PVP nanogels by irradiating a deoxygenated dilute aqueous solution of the polymer with a very high dose rate of electrons in pulse mode. As PVP does not contain any functional group that is sensitive towards solvated electrons, the sample was thus saturated with nitrous oxide to convert the solvated electron into hydroxyl radicals. As proven by the researchers, the PVP reacted with hydroxyl radicals by means of hydrogen abstraction. They reported that there was no change in molecular weight, but the size of the gel decreased, thus proving the formation of nanogels and intramolecular crosslinking [89].
Henke et al. synthesized Polyvinylpyrrolidone (PVP) and PAA nanogels in their study. They first prepared PVP and PAA complexes to obtain a certain molar fraction of carboxylic groups. The mixture was then irradiated in a glass vessel under fast stirring with electrons in pulses [106]. The PVP and PAA mixture was kept at a low concentration to avoid intermolecular crosslinking. They reported that there was a small increase in molecular weight for the irradiated mixture in comparison to the irradiated pure PVP. The small increase in molecular weight was the result of some intermolecular crosslinking that occurred simultaneously with the dominant intramolecular crosslinking processes. The intrinsic viscosity and radius of gyration of the microgels decreased at higher doses, suggesting evidence of formation of tightly crosslinked nanogels.
PVP nanogels were synthesized by intra molecular crosslinking via pulse radiolysis. PVP solutions were prepared by mixing PVP monomer, NaCl, and KCl in a buffer solution at a pH of 7.4 [107]. PVP can also be grafted to AA using high-energy electron beam irradiation to synthesize nanogels. They reported that nanogels with controlled size distribution and functionality was achieved using this method [78,108]. Hybrid nanogels can also be synthesized using an irradiation-induced method. For example, in one study, temperature-responsive nanogels were prepared via the immobilization of quantum dots in the interior spaces of nanogels [25].
Apart from chemical initiators, emulsion polymerization can also be initiated using ionizing radiation. Song et al. demonstrated that nanogels could be produced via the irradiation of styrene microemulsion. The size of the polystyrene (PS) gel obtained ranged from 52 to 210 nm [109]. The same application of ionizing radiation can also be used on inverse micelle systems, as reported by Yusof et al. [22] in their synthesis of PEGDA nanogels. In their work, a radiation crosslinkable polymer PEGDA solution, n heptane, and dioctyl sulfosuccinate (AOT) were first mixed. Then, the mixture was stirred until a clear one-phase solution formed and then exposed to an electron beam. The emulsion proved to be a suitable template for the polymerization of water-soluble crosslinkable polymers such as PEGDA (Fig. 6 a). Upon irradiation of a ternary system, the energy imparted will be absorbed by the oil phase and scavenged by the micelles to produce a hydroxyl radical via the direct radiolysis of water (b). Polymeric macroradicals are then formed due to hydroxyl radical attack (c). Macroradicals that are generated inside the micelle then recombine to form nanogels in the micelle (d).
a Entrapped PEGDA and water in AOT micelles. b Energy imparted is absorbed by the oil phase, producing an excess of electrons, which are scavenged by the micelles in the system to produce hydroxyl radicals via the direct radiolysis of water. c Polymeric macroradicals are formed due to an attack from the hydroxyl radicals. d Macroradicals recombine to form nanogels in the micelle. Reproduced with permission from [22]
Several works involving surfactant-free irradiation methods have been presented in the past including [50, 77, 89, 110, 111]. All these works have a disadvantage in terms of controlling the molecular weight and size of the final product. In 2012, Kadlubowski et al. suggested a new method that would allow for the control of the size and molecular weight of a gel [112]. In this new method, nanogels from PVP were synthesized in two stages (Fig. 7).
The two-stage synthesis of nanogels is shown. a Initially, PVP was prepared to a concentration above its critical hydrodynamic concentration, b With irradiation at a dose lower than the gelation dose DG, an increase in molecular weight and size was observed. c Continuous irradiation from (b) exceeding DG yielded wall-to-wall gel. d Change of mode of irradiation from low dose rates of gamma irradiation to pulses of fast electron yielded nanogels with controlled molecular weight and size. Reproduced with permission from [112]
In the first stage, a PVP solution was prepared to a concentration above its critical hydrodynamic concentration. The solution was then irradiated at a low dose rate to yield PVP microradicals at 10−7 mol dm−3 of concentration, in a system with a much higher amount of polymer coils than the microradical concentration value with the amount of average radicals per macromolecule being lower than 1. Under this condition, the macromolecules in the system would prefer intermolecular crosslinking, which would lead to an increase in size and molecular weight. The second stage of the process was done to stop further growth of the macromolecules. The above-mentioned irradiated solution was then diluted to a concentration lower than its coil overlapping concentration and then subjected to pulses of fast electron. Short and intense pulses of fast electrons generated radical concentrations of around 10−4–10−3 mol dm−3. Such a high value in a diluted macromolecules solution may result in the simultaneous creation of many radicals on each of the micromolecule. Under this condition, intramolecular crosslinking is preferable, at which point nanogels with almost constant molecular weight are formed regardless of dose increment. By adjusting the initial dose during gamma irradiation followed with dilution and exposure to pulse irradiation, nanogels with certain molecular weight and size can readily be obtained (Fig. 8).
Changes in molecular weight of PVP as a function of absorbed dose, ▲ 0.4 mol dm−3 PVP sample irradiated with gamma ray at a dose rate of 1.5 kGy h−1, ● Initial gamma irradiation was 300Gy followed by pulses of electrons, ■ Initial gamma irradiation was 500Gy followed by pulses of electrons. All samples were saturated with oxygen-free N2O prior and during irradiation. ●, ■ were diluted from ▲ prior to electron pulse irradiation [92]
Irradiation-induced synthesis can also be used to synthesize nanoparticles from natural polymers. Although the normal response of natural polymers towards ionizing irradiation is fragmentation and aggregation, under certain processing conditions, crosslinking to form nanoparticles can be achieved. In one study, protein nanogels were prepared via the gamma irradiation of bovine serum albumin (BSA) as the basic material [23, 113, 114]. The BSA was dissolved in ethanol solution, and then irradiated with gamma rays at a low dose rate at a relatively low temperature range. It was found that different concentrations of ethanol in the albumin solution led to different-sized nanogels.
The radiation-induced synthesis of nanogels can also be combined with other controlled-radical polymerization such as the Reversible Addition–Fragmentation Chain Transfer (RAFT) method to achieve narrow molecular weight distributions, controlled molecular weights, and complex architectures. Recently, the PNIPAAM nanogel was prepared using two RAFT agents in dimethylformamide (DMF). The polymerization was initiated using gamma rays [115]. Amphiphilic copolymers containing temperature and pH sensitive units with hydrophobic cores were produced using the same combination of methods [29].
Apart from being able to produce submicron gels from reactants in solution form, radiation-induced polymerization is also useful in the preparation of microgels from bulk materials. In a Brabender mixing chamber coupled with an electron beam setup, thermoplastic microgels from polypropylene (PP) and ethylene octene copolymer (EOC) were prepared via electron-induced reactive processing. Briefly, equal amounts of PP and EOC were melted and mixed in a mixing chamber at a speed of 45 rpm and at 180 °C in the presence of air. The mixing process was accompanied with simultaneous exposure to electron beams at 100 kGy with electron energy of 1.5 MeV. The microgels were used as an additive material in a thermoplastic matrix to improve elasticity and melt processability [21]. Recent report related to this type of synthesis method can be found in the work of Cruz et al. [116]. Summary of selected methods discussed under this subsection can be found in Table 7.
The above review indicates that the irradiation route is obviously an attractive method in producing submicron gels, as the process normally comprises a single-step technique. Also, in some cases, the doses applied are equivalent to a sterilization dose. Therefore, in addition to polymerization, the irradiated samples are also sterilized. Nevertheless, there is one disadvantage to this method—although it is facility-dependent, the availability of ionizing irradiators is still low and access to similar services is also limited. Table 8
gives a summary in terms of methods, materials, advantages and disadvantages of methods from different categories discussed in this review.
Conclusion
This review presents an overview of the basic aspects regarding the definition, classification, and synthesis methods of organic submicron gels. Submicron gels have undergone interesting transformations from being an unwanted by-product to now being applied in many emerging advanced applications. The emergence of new applications, meanwhile, has driven the discovery of new technique of syntheses that allow for precise tailoring of the final products. Submicron gels can be synthesized using emulsion-based crosslinking, lithographic methods, and irradiation-induced methods—each with its own strengths, advantages, and disadvantages. The selection of methods can sometimes be limited to the properties of the reactant (i.e. water solubility, economic, application, facility, etc.). Nevertheless, these methods can also be combined to complement each other, resulting in a far superior process. In summary, one would find that the best combination of processing techniques would comprise a one-pot aqueous setup coupled with irradiation-induced polymerization/crosslinking on a polymer solution as the starting material. This setup would allow for the highly advantageous processing of submicron gels.
References
Raemdonck K, Braeckmans K, Demeester J, De Smedt SC (2014) Merging the best of both worlds: hybrid lipid-enveloped matrix nanocomposites in drug delivery Chem Soc Rev 43:444–472
Islam MR, Gao Y, Li X, Serpe MJ (2014) Responsive polymers for biosensing and protein delivery J Mater Chem B 2:2444–2451
Krasia-Christoforou T, Georgiou TK (2013) Polymeric theranostics: using polymer-based systems for simultaneous imaging and therapy J Mater Chem B 1:3002–3025
Mimi H, Ho KM, Siu YS, Wu A, Li P (2012) Polyethyleneimine-based core-shell nanogels: a promising siRNA carrier for argininosuccinate synthetase mRNA knockdown in HeLa cells J Control Release 158:123–130
Park CW, Yang H-M, Lee HJ, Kim J-D (2013) Core-shell nanogel of PEG-poly (aspartic acid) and its pH-responsive release of rh-insulin Soft Matter 9:1781–1788
Qian H, Wang X, Yuan K, Xie C, Wu W, Jiang X, Hu L (2014) Delivery of doxorubicin in vitro and in vivo using bio-reductive cellulose nanogels Biomaterials Science 2:220–232
Yoo J-W, Doshi N, Mitragotri S (2011) Adaptive micro and nanoparticles: temporal control over carrier properties to facilitate drug delivery Adv Drug Deliv Rev 63:1247–1256
Elsabahy M, Wooley KL (2012) Design of polymeric nanoparticles for biomedical delivery applications Chem Soc Rev 41:2545–2561
Caruso F, Hyeon T, Rotello VM (2012) Nanomedicine Chem Soc Rev 41:2537–2538
Malmsten M, Bysell H, Hansson P (2010) Biomacromolecules in microgels—opportunities and challenges for drug delivery Curr Opin Colloid Interface Sci 15:435–444
Fan YF, Wang YN, Fan YG, Ma JB (2006) Preparation of insulin nanoparticles and their encapsulation with biodegradable polyelectrolytes via the layer-by-layer adsorption Int J Pharm 324:158–167
Prakash S, Martoni C (2006) Toward a new generation of therapeutics Appl Biochem Biotechnol 128:1–21
Alemán J, Chadwick AV, He J, et al. (2007) Definitions of terms relating to the structure and processing of sols, gels, networks, and inorganic-organic hybrid materials (IUPAC Recommendations 2007) Pure Appl Chem 79:1801–1829
Wagner V, Dullaart A, Bock A-K, Zweck A (2006) The emerging nanomedicine landscape Nat Biotechnol 24:1211–1217
Ulanski P, Rosiak JM (2004) Polymeric nano/microgels. Encyclopedia of nanoscience and nanotechnology. American Scientific Publishers, pp 845–871
Peppas NA, Huang Y (2004) Nanoscale technology of mucoadhesive interactions Adv Drug Deliv Rev 56:1675–1687
Fathi M, Martin A, McClements DJ (2014) Nanoencapsulation of food ingredients using carbohydrate based delivery systems Trends Food Sci Technol 39:18–39
Rinaudo M (2006) Chitin and chitosan: properties and applications Prog Polym Sci 31:603–632
Chen Y, Ballard N, Bon SA (2013) Waterborne polymer nanogels non-covalently crosslinked by multiple hydrogen bond arrays Polym Chem 4:387–392
Jiang M, Li M, Xiang M, Zhou H (1999) Interpolymer complexation and miscibility enhancement by hydrogen bonding. Polymer Synthesis/Polymer-Polymer Complexation. Springer, pp 121–196
Rajeshbabu R, Gohs U, Naskar K, Thakur V, Wagenknecht U, Heinrich G (2011) Preparation of polypropylene (PP)/ethylene octene copolymer (EOC) thermoplastic vulcanizates (TPVs) by high energy electron reactive processing Radiat Phys Chem 80:1398–1405
Hamzah Y, Yunus WMZW, Isa NM, Tajau R, Hashim K, Dahlan KZ (2012) Synthesis of polyethylene glycol diacrylate nanogel using irradiation of inverse micelles technique E-Polymers 12:533–538
Espinoza SLS, Sánchez ML, Risso V, Smolko EE, Grasselli M (2012) Radiation synthesis of seroalbumin nanoparticles Radiat Phys Chem 81:1417–1421
Zhang X, Malhotra S, Molina M, Haag R (2015) Micro-and nanogels with labile crosslinks-from synthesis to biomedical applications Chem Soc Rev 44:1948–1973
Zhu H, Li Y, Qiu R, Shi L, Wu W, Zhou S (2012) Responsive fluorescent Bi 2 O 3@ PVA hybrid nanogels for temperature-sensing, dual-modal imaging, and drug delivery Biomaterials 33:3058–3069
Singh S, Mӧller M, Pich A (2013) Biohybrid nanogels J Polym Sci A Polym Chem 51:3044–3057
Storm G, Belliot SO, Daemen T, Lasic DD (1995) Surface modification of nanoparticles to oppose uptake by the mononuclear phagocyte system Adv Drug Deliv Rev Elsevier 17:31–48
Shaik MR, Korsapati M, Panati D (2012) Polymers in controlled drug delivery systems Int J Pharma Sci 2:112–116
Picos-Corrales LA, Licea-Claverie A, Arndt K-F (2014) Bisensitive core-shell nanohydrogels by e-Beam irradiation of micelles React Funct Polym. Elsevier 75:31–40
Kumar A, Lahiri SS, Singh H (2006) Development of PEGDMA: MAA based hydrogel microparticles for oral insulin delivery Int J Pharm Elsevier 323:117–124
Mauri E, Moroni I, Magagnin L, Masi M, Sacchetti A, Rossi F (2016) Comparison between two different click strategies to synthesize fluorescent nanogels for therapeutic applications React Funct Polym. Elsevier 105:35–44
Sahiner N, Ozay O, Aktas N, Blake DA, John VT (2011) Arsenic (V) removal with modifiable bulk and nano p (4-vinylpyridine)-based hydrogels: the effect of hydrogel sizes and quarternization agents Desalination. Elsevier 279:344–352
Candau F, Leong YS, Pouyet G, Candau S (1984) Inverse microemulsion polymerization of acrylamide: characterization of the water-in-oil microemulsions and the final microlatexes J Colloid Interface Sci Elsevier 101:167–183
Landfester K, Pawelzik U, Antonietti M (2005) Polydimethylsiloxane latexes and copolymers by polymerization and polyaddition in miniemulsion Polymer. Elsevier 46:9892–9898
Salehi P, Makhoul G, Roy R, Malhotra M, Mood ZA, Daniel SJ (2013) Curcumin loaded NIPAAM/VP/PEG-A nanoparticles: physicochemical and chemopreventive properties J Biomater Sci Polym Ed. Taylor & Francis 24:574–588
Deen GR, Alsted T, Richtering W, Pedersen JS (2011) Synthesis and characterization of nanogels of poly (N-isopropylacrylamide) by a combination of light and small-angle X-ray scattering Phys Chem Chem Phys R Soc Chem 13:3108–3114
Missirlis D, Hubbell J, Tirelli N (2006) Thermally-induced glass formation from hydrogel nanoparticles Soft Matter R Soc Chem 2:1067–1075
Peng J, Qi T, Liao J, Fan M, Luo F, Li H, Qian Z (2012) Synthesis and characterization of novel dualresponsive nanogels and their application as drug delivery systems Nanoscale R Soc Chem 4:2694–2704
Crespy D, Zuber S, Turshatov A, Landfester K, Popa A-M (2012) A straightforward synthesis of fluorescent and temperature-responsive nanogels J Polym Sci A Polym Chem Wiley Online Library 50:1043–1048
Wang Y, Zheng J, Tian Y, Yang W (2015) Acid degradable poly (vinylcaprolactam)-based nanogels with ketal linkages for drug delivery J Mater Chem B R Soc Chem 3:5824–5832
Medeiros SF, Santos AM, Fessi H, Elaissari A (2010) Synthesis of biocompatible and thermally sensitive poly (N-vinylcaprolactam) nanogels via inverse miniemulsion polymerization: Effect of the surfactant concentration J Polym Sci A Polym Chem Wiley Online Library 48:3932–3941
Tian Y, Bian S, Yang W (2016) A redox-labile poly (oligo (ethylene glycol) methacrylate)-based nanogel with tunable thermosensitivity for drug delivery Polym Chem R Soc Chem 7:1913–1921
Lou S, Gao S, Wang W, Zhang M, Zhang J, Wang C, Li C, Kong D, Zhao Q (2015) Galactosefunctionalized multi-responsive nanogels for hepatoma-targeted drug delivery Nanoscale R Soc Chem 7:3137–3146
Sengel SB, Sahiner N (2016) Poly (vinyl phosphonic acid) nanogels with tailored properties and their use for biomedical and environmental applications Eur Polym J. Elsevier 75:264–275
Yi P, Wang Y, Zhang S, Zhan Y, Zhang Y, Sun Z, Li Y, He P (2017) Stimulative nanogels with enhanced thermosensitivity for therapeutic delivery via beta-cyclodextrin-induced formation of inclusion complexes Carbohydr Polym. Elsevier 166:219–227
Yun YH, Goetz DJ, Yellen P, Chen W (2004) Hyaluronan microspheres for sustained gene delivery and site-specific targeting Biomaterials. Elsevier 25:147–157
Singh S, Blӧhbaum J, Mӧller M, Pich A (2012) Biohybrid nanogels by crosslinking of ovalbumin with reactive star-PEGs in W/O emulsions J Polym Sci A Polym Chem Wiley Online Library 50:4288–4299
Kumbar S, Kulkarni A, Aminabhavi T (2002) Crosslinked chitosan microspheres for encapsulation of diclofenac sodium: effect of crosslinking agent. Journal of microencapsulation Informa UK Ltd UK 19:173–180
Zhang J, Lu Z, Wu M, Wu Q, Yang J (2015) Large-scale synthesis and characterization of magnetic poly (acrylic acid) nanogels via miniemulsion polymerization RSC Adv R Soc Chem 5:58889–58894
Ulanski P, Kadlubowski S, Rosiak JM (2002) Synthesis of poly (acrylic acid) nanogels by preparative pulse radiolysis Radiat Phys Chem. Elsevier 63:533–537
Sahiner N, Demirci S (2016) PEI-based hydrogels with different morphology and sizes: Bulkgel, microgel, and cryogel for catalytic energy and environmental catalytic applications Eur Polym J. Elsevier 76:156–169
Su H, Jia Q, Shan S (2016) Synthesis and characterization of Schiff base contained dextran microgels in water-in-oil inverse microemulsion Carbohydr Polym. Elsevier 152:156–162
Hsiao L-W, Lai Y-D, Lai J-T, Hsu C-C, Wang N-Y, Steven S-SW, Jan J-S (2017) Cross-linked polypeptide-based gel particles by emulsion for efficient protein encapsulation. Polymer. Elsevier
Sun Y, Lyu X, Li Z, Huang Y (2017) Guanidinium functionalized polypeptide nanogels as the phosphate binder Polymer. Elsevier 112:325–332
Zhang Y-X, Chen Y-F, Shen X-Y, Hu J-J, Jan J-S (2016) Reduction-and pH-Sensitive lipoic acidmodified Poly (l-lysine) and polypeptide/silica hybrid hydrogels/nanogels Polymer. Elsevier 86:32–41
Singka GSL, Samah NA, Zulfakar MH, Yurdasiper A, Heard CM (2010) Enhanced topical delivery and anti-inflammatory activity of methotrexate from an activated nanogel Eur J Pharm Biopharm Elsevier 76:275–281
Zhou Y, Tang H, Wu P (2015) Volume phase transition mechanism of poly [oligo (ethylene glycol) methacrylate] based thermo-responsive microgels with poly (ionic liquid) cross-linkers Phys Chem Chem Phys R Soc Chem 17:25525–25535
An Y, Zhang L, Xiong S, Wu S, Xu M, Xu Z (2012) Fluorine-containing thermo-sensitive microgels as carrier systems for biomacromolecules Colloids Surf B: Biointerfaces. Elsevier 92:246–253
Verma AK, Chanchal A, Maitra A (2010) Co-polymeric hydrophilic nanospheres for drug delivery: release kinetics, and cellular uptake Indian J Exp Biol 48:1043–1052
Zhao L, Xiao C, Ding J, He P, Tang Z, Pang X, Zhuang X, Chen X (2013) Facile one-pot synthesis of glucose-sensitive nanogel via thiol-ene click chemistry for self-regulated drug delivery Acta Biomater. Elsevier 9:6535–6543
Ramos J, Hidalgo-Alvarez R, Forcada J (2013) Facile synthesis of thermoresponsive nanohybrids Soft Matter R Soc Chem 9:8415–8419
Yao R-S, Zhang W, Yang X-Z, Liu J, Liu H-T (2014) HPMC/PAA hybrid nanogels via aqueous-phase synthesis for controlled delivery of insulin Biomater Sci R Soc Chem 2:1761–1767
Thaiboonrod S, Berkland C, Milani AH, Ulijn R, Saunders BR (2013) Poly (vinylamine) microgels: pH-responsive particles with high primary amine contents Soft Matter R Soc Chem 9:3920–3930
Zhao D, Shi X, Liu T, Lu X, Qiu G, Shea KJ (2016) Synthesis of surfactant-free hydroxypropyl methylcellulose nanogels for controlled release of insulin Carbohydr Polym. Elsevier 151:1006–1011
Abreu CM, Paula HC, Seabra V, Feitosa JP, Sarmento B, de Paula RC (2016) Synthesis and characterization of non-toxic and thermo-sensitive poly (N-isopropylacrylamide)-grafted cashew gum nanoparticles as a potential epirubicin delivery matrix Carbohydr Polym. Elsevier 154:77–85
Rolland JP, Maynor BW, Euliss LE, Exner AE, Denison GM, DeSimone JM (2005) Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials J Am Chem Soc ACS Publications 127:10096–10100
Zhang H, Tumarkin E, Sullan RMA, Walker GC, Kumacheva E (2007) Exploring microfluidic routes to microgels of biological polymers Macromol Rapid Commun. Wiley Online Library 28:527–538
Steinhilber D, Rossow T, Wedepohl S, Paulus F, Seiffert S, Haag R (2013) A microgel construction kit for bioorthogonal encapsulation and ph-controlled release of living cells Angew Chem Int Ed. Wiley Online Library 52:13538–13543
Zhao C, Chen Q, Patel K, Li L, Li X, Wang Q, Zhang G, Zheng J (2012) Synthesis and characterization of pH-sensitive poly (N-2-hydroxyethyl acrylamide)-acrylic acid (poly (HEAA/AA)) nanogels with antifouling protection for controlled release Soft Matter R Soc Chem 8:7848–7857
Jia X, Yeo Y, Clifton RJ, Jiao T, Kohane DS, Kobler JB, Zeitels SM, Langer R (2006) Hyaluronic acidbased microgels and microgel networks for vocal fold regeneration Biomacromolecules ACS Publications 7:3336–3344
Berger S, Ornatsky O, Baranov V, Winnik MA, Pich A (2010) Hybrid nanogels by encapsulation of lanthanide-doped LaF 3 nanoparticles as elemental tags for detection by atomic mass spectrometry J Mater Chem R Soc Chem 20:5141–5150
Tang S, Shi Z, Cao Y, He W (2013) Facile aqueous-phase synthesis of multi-responsive nanogels based on polyetheramines and bisepoxide J Mater Chem B R Soc Chem 1:1628–1634
Wang B, Hong Y, Feng J, Gong Y, Gao C (2007) Rings of Hydrogel Fabricated by a Micro-Transfer Technique Macromol Rapid Commun. Wiley Online Library 28:567–571
Karabacak RB, Türk H (2012) Preparation of PS Colloids with DMA and MMA Comonomers and Suitability of P (S/DMA) for Colloidal Silica Deposition J Macromol Sci A. Taylor & Francis 49:680–688
Song J-S, Tronc F, Winnik MA (2004) Two-stage dispersion polymerization toward monodisperse, controlled micrometer-sized copolymer particles J Am Chem Soc ACS Publications 126:6562–6563
Woods HM, Nouvel C, Licence P, Irvine DJ, Howdle SM (2005) Dispersion polymerization of methyl methacrylate in supercritical carbon dioxide: an investigation into stabilizer anchor group Macromolecules. ACS Publications 38:3271–3282
Dispenza C, Grimaldi N, Sabatino M-A, Todaro S, Bulone D, Giacomazza D, Przybytniak G, Alessi S, Spadaro G (2012) Studies of network organization and dynamics of e-beam crosslinked PVPs: From macro to nano Radiat Phys Chem. Elsevier 81:1349–1353
El-Rehim HAA, Hegazy E-SA, Diaa DA (2012) Photo-catalytic degradation of Metanil Yellow dye using TiO 2 immobilized into polyvinyl alcohol/acrylic acid microgels prepared by ionizing radiation React Funct Polym. Elsevier 72:823–831
Vinogradov SV, Bronich TK, Kabanov AV (2002) Nanosized cationic hydrogels for drug delivery: preparation, properties and interactions with cells Adv Drug Deliv Rev. Elsevier 54:135–147
Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Ipe BI, Bawendi MG, Frangioni JV (2007) Renal clearance of quantum dots Nat Biotechnol Nat Publ Group 25:1165–1170
Longmire M, Choyke PL, Kobayashi H (2008) Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Future Medicine
Barry BW (2001) Novel mechanisms and devices to enable successful transdermal drug delivery Eur J Pharm Sci. Elsevier 14:101–114
Brannon-Peppas L, Blanchette JO (2004) Nanoparticle and targeted systems for cancer therapy Adv Drug Deliv Rev. Elsevier 56:1649–1659
Kievit FM, Zhang M (2011) Cancer nanotheranostics: improving imaging and therapy by targeted delivery across biological barriers Adv Mater. Wiley Online Library 23
Asua JM (2002) Miniemulsion polymerization Prog Polym Sci. Elsevier 27:1283–1346
Yong CP, Gan LM (2005) Microemulsion polymerizations and reactions. Polymer Particles. Springer. p 257–298
Lu Y, Ballauff M (2011) Thermosensitive core-shell microgels: from colloidal model systems to nanoreactors. Progress in Polymer Science. Elsevier. p 767–792
Wei J, Yu Y (2012) Photodeformable polymer gels and crosslinked liquid-crystalline polymers Soft Matter R Soc Chem 8:8050–8059
Ulanski P, Janik I, Rosiak J (1998) Radiation formation of polymeric nanogels Radiat Phys Chem Elsevier 52:289–294
Yusof H, Naurah MI, Liyana MAN (2014) Polyethylene glycol diacrylate microgels from irradiated micelles. Advanced Materials Research. p 316–319
Sjӧblom J, Lindberg R, Friberg SE (1996) Microemulsions—phase equilibria characterization, structures, applications and chemical reactions Adv Colloid Interf Sci. Elsevier 65:125–287
Antonietti M, Landfester K (2002) Polyreactions in miniemulsions Prog Polym Sci Elsevier 27:689–757
Zhuang J, Gordon MR, Ventura J, Li L, Thayumanavan S (2013) Multi-stimuli responsive macromolecules and their assemblies Chem Soc Rev R Soc Chem 42:7421–7435
Dyab AK, Atta AM (2013) Microgel-stabilised non-aqueous emulsions RSC Adv R Soc Chem 3:25662–25665
McClements DJ (2012) Nanoemulsions versus microemulsions: terminology, differences, and similarities Soft Matter R Soc Chem 8:1719–1729
Walz R, Bӧmer B, Heitz W (1977) Monomeric and polymeric azoinitiators Die Makromol Chem. Wiley Online Library 178:2527–2534
Pang HT, Chen XG, Park HJ, Cha DS, Kennedy JF (2007) Preparation and rheological properties of deoxycholate-chitosan and carboxymethyl-chitosan in aqueous systems Carbohydr Polym. Elsevier 69:419–425
Leong YS, Candau F (1982) Inverse microemulsion polymerization J Phys Chem. ACS Publications 86:2269–2271
Fang S-J, Fujimoto K, Kondo S, Shiraki K, Kawaguchi H (2001) Amphoteric initiators suitable for emulsifier-free emulsion polymerization and the properties of the resulting latices Colloid Polym Sci. Springer 279:589–596
Fang S-J, Fujimoto K, Kondo S, Shiraki K, Kawaguchi H (2000) Emulsifier-free emulsion copolymerization of styrene and acrylamide using an amphoteric initiator Colloid Polym Sci Springer 278:864–871
Wang Z-K, Wang L-H, Sun J-T, Han L-F, Hong C-Y (2013) In situ generation of bioreducible and acid labile nanogels/microgels simply via adding water into the polymerization system Poly Chem R Soc Chem 4:1694–1699
Liu Y-Y, Liu X-Y, Yang J-M, Lin D-L, Chen X, Zha L-S (2012) Investigation of Ag nanoparticles loading temperature responsive hybrid microgels and their temperature controlled catalytic activity. Colloids and Surfaces A: Physicochemical and Engineering Aspects. Elsevier 393:105–110
Xue Y, Xia X, Yu B, Luo X, Cai N, Long S, Yu F (2015) A green and facile method for the preparation of a pH-responsive alginate nanogel for subcellular delivery of doxorubicin Adv R Soc Chem 5:73416–73423
Nie Z, Li W, Seo M, Xu S, Kumacheva E (2006) Janus and ternary particles generated by microfluidic synthesis: design, synthesis, and self-assembly J Am Chem Soc ACS Publications 128:9408–9412
Brasch U, Burchard W (1996) Preparation and solution properties of microhydrogels from poly (vinyl alcohol) Macromol Chem Phys. Wiley Online Library 197:223–235
Henke A, Kadlubowski S, Ulanski P, Rosiak JM, Arndt K-F (2005) Radiation-induced cross-linking of polyvinylpyrrolidone-poly (acrylic acid) complexes Nucl Instrum Methods Phys Res, Sect B Elsevier 236:391–398
An J-C, Weaver A, Kim B, Barkatt A, Poster D, Vreeland WN, Silverman J, Al-Sheikhly M (2011) Radiation-induced synthesis of poly (vinylpyrrolidone) nanogel Polymer Elsevier 52:5746–5755
Grimaldi N, Sabatino M, Przybytniak G, Kaluska I, Bondi M, Bulone D, Alessi S, Spadaro G, Dispenza C (2014) High-energy radiation processing, a smart approach to obtain PVP-graft-AA nanogels Radiat Phys Chem. Elsevier 94:76–79
Song L, Wang M, Cong Y, Liu W, Ge X, Zhang Z (2007) The mechanism of Co-60 gamma ray radiation induced interfacial redox reaction in inverse emulsion and its application in the synthesis of polymer microcapsules Polymer. Elsevier 48:150–157
Kadlubowski S, Grobelny J, Olejniczak W, Cichomski M, Ulanski P (2003) Pulses of fast electrons as a tool to synthesize poly (acrylic acid) nanogels. Intramolecular cross-linking of linear polymer chains in additive-free aqueous solution Macromolecules ACS Publications 36:2484–2492
Ulanski P, Rosiak J (1999) The use of radiation technique in the synthesis of polymeric nanogels Nucl Instrum Methods Phys Res, Sect B Elsevier 151:356–360
Kadlubowski S, Ulanski P, Rosiak JM (2012) Synthesis of tailored nanogels by means of two-stage irradiation Polymer. Elsevier 53:1985–1991
Achilli E, Casajus G, Siri M, Flores C, Kadlubowski S, del Alonso SV, Grasselli M (2015) Preparation of protein nanoparticle by dynamic aggregation and ionizing-induced crosslinking Colloids Surf A Physicochem Eng Asp. Elsevier 486:161–171
Varca GH, Ferraz CC, Lopes PS, Beatriz Mathor M, Grasselli M, Lugão AB (2014) Radio-synthesized protein-based nanoparticles for biomedical purposes Radiat Phys Chem. Elsevier 94:181–185
Kiraç F, Güven O (2015) Gamma radiation induced synthesis of poly (N-isopropylacrylamide) mediated by Reversible Addition-Fragmentation Chain Transfer (RAFT) process Radiat Phys Chem. Elsevier 112:76–82
Cruz A, Garcia-Uriostegui L, Ortega A, Isoshima T, Burillo G (2017) Radiation grafting of Nvinylcaprolactam onto nano and macrogels of chitosan: Synthesis and characterization Carbohydr Polym. Elsevier 155:303–312