Abstract
The aim of this study was to investigate the effectiveness of instruction supplemented by conceptual change texts (CCTs) over traditional instruction on students’ understanding of electrochemical (galvanic and electrolytic) cell concepts. The participants of the study consisted of 64 students from the two classes of a high school located in Turkey. Classes were randomly assigned to experimental group, which was exposed to CCTs as a supplementary material, and to control group, which was exposed to traditional instruction. A 23-item multiple-choice test was developed assess students’ conceptual understanding of electrochemical cells. This test was administered to both groups before and after the instruction. The results of ANCOVA indicated that students who were instructed by using CCTs had better conceptual understanding of electrochemical cells than those experiencing traditional instruction when their prior electrochemical cell concepts understanding was statistically controlled. The findings of this study suggest that CCTs can be used as a cost- and resource-effective supplement to classroom instruction to promote students’ understanding science concepts.

Similar content being viewed by others
References
Allsop RT, George NH (1982) Redox in Nuffield advanced chemistry. Edu Chem 10:57–59
Alvermann DE, Hynd CR (1989) Effects of prior knowledge activation modes and text structure of nonscience majors’ comprehension of physics. J Edu Res 83:97–102
Butts B, Smith R (1987) What do students perceive as difficult in HSC chemistry? Aus Sci Teachers J 32:45–51
Chambers KS, Andre T (1997) Gender, prior knowledge, interest, and experience in electricity and conceptual change text manipulations in learning about direct current. J Res Sci Teaching 34:107–123
Champagne A, Gunstone R, Klopfer L (1985) Effecting changes in cognitive structures among physical students. In: West, L, Pines L (eds) Cognitive structure and conceptual change. Academic Press, London
Diakidoy IN, Kendeou P, Ioannides C (2003) Reading about energy: the effects of text structure in science learning and conceptual change. Contemp Edu Psychol 28:335–356
Driver R, Easley J (1978) Pupils and paradigm: a review of literature related to the concept development in adolescent science students. Stud Sci Edu 5:61–84
Finley F, Stewart J, Yarroch W (1982) Teachers’ perceptions of important and difficult science content. Sci Edu 66(4):531–538
Garnett PJ, Treagust DF (1992) Conceptual difficulties experienced by senior high school students of electrochemistry: electrochemical (galvanic) and electrolytic cells. J Res Sci Teaching 29:1079–1099
Guzzetti BJ, Synder TE, Glass GV, Gamas WS (1993) Promoting conceptual change in science: a comparative meta-analysis of instructional interventions from reading education and science education. Read Res Q 28:116–159
Hennessey MG (1999). Probing the dimensions of metacognition: implications for conceptual change teaching-learning. Paper presented at the Annual Meeting of the National Association for Research in Science Teaching. Boston, MA
Hewson GM, Hewson WP (1983) Effect of instruction using students’ prior knowledge and conceptual change strategies on science learning. J Res Sci Teaching 20:731–743
Hewson PW, Thorley R (1989) The conditions of conceptual change in classroom. Int J Sci Edu 11:541–553
Hynd CR, McWhorter YJ, Phares VL, Suttles CW (1994) The role of instructional variables in conceptual change in high school physics topics. J Res Sci Teaching 31:933–946
Maria K, MacGinitie W (1987) Learning from texts that refute the reader’s prior knowledge. Read Res Instr 26:222–238
Nussbaum J, Novick S (1982) A study of conceptual change in the classroom. Paper presented in the National Association for Research in Science Teaching, Lake Geneva, Chicago
Ogude AN, Bradley JD (1994) Ionic conduction and electrical neutrality in operating electrochemical cells. J Chem Edu 71:29–31
Ogude AN, Bradley JD (1996) Electrode processes and aspects relating to cell emf, current, and cell components in operating electrochemical cells: pre-college and college student interpretation. J Chem Edu 71:29–31
Palmer DH (2003) Investigating the relationship between refutational text and conceptual change. Sci Edu 87:663–684
Posner GJ, Strike KA, Hewson PW, Gertzog WA (1982) Accommodation of a scientific conception: toward a theory of conceptual change. Sci Edu 66:211–227
Roth KS, Rosaen CL (1991) Investigating science concepts trough writing activities. Paper presented at the annual meeting of the National Association for Research in Science Teaching, Fontana, WI
Sanger MJ, Greenbowe TJ (1997) Common student misconceptions in electrochemistry: galvanic, electrolytic, and concentration cells. J Res Sci Teaching 34:377–398
Sanger MJ, Greenbowe TJ (2000) Addressing student misconceptions concerning electron flow in aqueous solutions with instruction including computer animations and conceptual change strategies. Int J Sci Edu 22:521–537
Scott PH, Asoko HM, Driver RH (1992) Teaching for conceptual change: a review of strategies. In: Duit R, Goldberg F, Niedderer H (eds) Research in physics learning: theoretical issues and empirical studies. Institute for Science Education at the University of Kiel, Kiel, Germany, pp 310–329
Smith LE, Blakeslee DT, Anderson WC (1993) Teaching strategies associated with conceptual change learning in science. J Res Sci Teaching 30:111–126
Vosniadou S, Brewer WF (1987) Theories of knowledge restructuring in development. Rev Edu Res 57:51–67
Yenilmez A, Tekkaya C (2006) Enhancing students’ understanding of photosynthesis and respiration in plant through conceptual change approach. J Sci Edu Technol 15:81–87
Zietsman AI, Hewson PW (1986) Effect of instruction using microcomputer simulations and conceptual change strategies on science learning. J Res Sci Teaching 23:27–39
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix A
Examples of the Electrochemical Cell Concepts Test
-
4.
In a galvanic cell constructed by Ni and Ag electrodes, Ni electrode is anode and Ag electrode is cathode. Which one of the following statements is TRUE about the charges of the electrodes of this cell?
-
(a)
Ni electrode is negatively charged because it releases electrons.
-
(b)
The positive sign of the Ag electrode indicates that Ag has higher reduction potential.
-
(c)
Ag electrode is negatively charged because it attracts electrons.
-
(d)
Ni electrode is positively charged because it attracts negatively charged anions.
-
(a)
-
6.
Which one of the following statements is CORRECT about the standard reduction potentials?
-
(a)
Standard reduction potentials can be measured independently without the use of other half- cell reactions with the known potentials.
-
(b)
Half-cell reactions with the positive standard reduction potentials are spontaneous.
-
(c)
All standard reduction potentials are measured relative to the standard hydrogen electrode.
-
(d)
The metal which has the most positive standard reduction potential is the most reactive.
-
(a)
-
15.
In an electrochemical cell, conduction through the electrolyte is due to:
-
(a)
electrons moving through the solution attached to the ions.
-
(b)
the movement of negative ions.
-
(c)
electrons moving through the solution from one electrode to the other.
-
(d)
the movement of both positive and negative ions.
-
(a)
-
16.
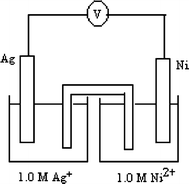 $$ {\text{Ag}}^{{\text{ + }}} {\text{(aq) + e}}^{{{-}}} \to {\text{ Ag(s),}}\quad {\text{ E}}^{{\text{o}}} {\text{ = + 0}}{\text{.80 V}} $$$$ {\text{Ni}}^{{{\text{2 + }}}} {\text{(aq) + 2e}}^{{{ - }}} {\text{ }} \to {\text{Ni(s),}}\quad {\text{ E}}^{{\text{o}}} {{ = {\text {-}}0}}{\text{.24 V}} $$
$$ {\text{Ag}}^{{\text{ + }}} {\text{(aq) + e}}^{{{-}}} \to {\text{ Ag(s),}}\quad {\text{ E}}^{{\text{o}}} {\text{ = + 0}}{\text{.80 V}} $$$$ {\text{Ni}}^{{{\text{2 + }}}} {\text{(aq) + 2e}}^{{{ - }}} {\text{ }} \to {\text{Ni(s),}}\quad {\text{ E}}^{{\text{o}}} {{ = {\text {-}}0}}{\text{.24 V}} $$In the electrochemical cell drawn above, electrons in the cell flow through the _____ toward the _______.
-
(a)
wire, silver electrode
-
(b)
wire, nickel electrode
-
(c)
wire, silver electrode and salt bridge, nickel electrode
-
(d)
wire, nickel electrode and salt bridge, silver electrode
-
(a)
-
18.
The function of a salt bridge in an electrochemical cell is to:
-
(a)
form complex ions with the oxidation products.
-
(b)
permit electrons to flow through the solution.
-
(c)
keep the levels of liquids equal in both half-cells.
-
(d)
allow positive and negative ions to enter and leave both half-cells.
-
(a)
-
19.
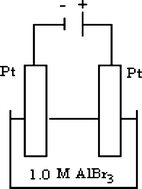
What are the products at the anode and cathode in the above electrolytic cell? (Al3+, H2O, Br2, O2 are arranged in the order of increasing standard reduction potentials in the following way:
$$ {\text{Al}}^{{{\text{3 + }}}} {\text{ $ < $ H}}_{{\text{2}}} {\text{O $ < $ Br}}_{{\text{2}}} {\text{ $ < $ O}}_{{\text{2}}} {\text{)}}{\text{.}} $$Anode
Cathode
(a) HBr
Al2O3
(b) Br2
H2
(c) Br2
Al
(d) O2
Al
-
20.
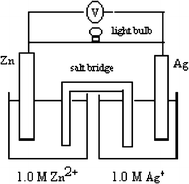
Evaluate the following assertion and reason listed below:
Assertion
Reason
If the salt bridge in the picture above was replaced by a copper wire (an electrical conductor), the light bulb would be lit.
...there will be a continuous flow of electrons in the electrolyte solutions that can pass through the copper bridge.
(a) Both the assertion and the reason are correct.
(b) The assertion is correct, but the reason is incorrect.
(c) The assertion is incorrect, but the reason is correct.
(d) Both the assertion and the reason are incorrect.
-
22.
Which drawing best describes the current flow occurring at the salt bridge in the Pb(NO3)2 solution?
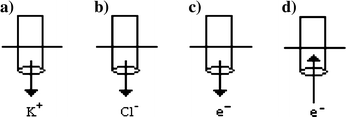
-
23.
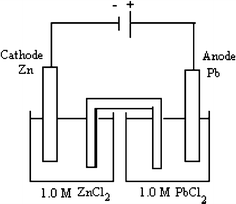 $$ {\text{Pb}}^{{{\text{2 + }}}} {\text{(aq) + 2e}}^{{\text{ - }}} {\text{ }} \to {\text{ Pb,}}\quad {\text{ E}}^{{\text{o}}} {\text{ = {\text {-}} 0}}{\text{.12 V}} $$$$ {\text{Zn}}^{{{\text{2 + }}}} {\text{(aq) + 2e}}^{{\text{ - }}} {\text{ }} \to {\text{ Zn,}}\quad {\text{ E}}^{{\text{o}}} {\text{ = {\text {-}}0}}{\text{.76 V}} $$
$$ {\text{Pb}}^{{{\text{2 + }}}} {\text{(aq) + 2e}}^{{\text{ - }}} {\text{ }} \to {\text{ Pb,}}\quad {\text{ E}}^{{\text{o}}} {\text{ = {\text {-}} 0}}{\text{.12 V}} $$$$ {\text{Zn}}^{{{\text{2 + }}}} {\text{(aq) + 2e}}^{{\text{ - }}} {\text{ }} \to {\text{ Zn,}}\quad {\text{ E}}^{{\text{o}}} {\text{ = {\text {-}}0}}{\text{.76 V}} $$What is the cell potential for the above electrolytic cell? (a) +0.88 V (b) −0.88 V (c) +0.64 V (d) −0.64 V
Appendix B
Understanding the Current Flow in Galvanic and Electrolytic Cells


Before reading the text below, write your answer for the above questions and your reasons in the empty space provided below. If your answer is not correct you may have misconceptions. In that case read the text more than once.

Misconception About Understanding the Current Flow in Galvanic Cells


Rights and permissions
About this article
Cite this article
Yürük, N. The Effect of Supplementing Instruction with Conceptual Change Texts on Students’ Conceptions of Electrochemical Cells. J Sci Educ Technol 16, 515–523 (2007). https://doi.org/10.1007/s10956-007-9076-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10956-007-9076-0









