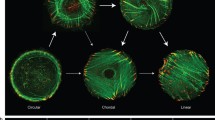
Abstract
I consider the microscopic mechanisms by which a particular left-right (L/R) asymmetry is generated at the organism level from the microscopic handedness of cytoskeletal molecules. In light of a fundamental symmetry principle, the typical pattern-formation mechanisms of diffusion plus regulation cannot implement the “right-hand rule”; at the microscopic level, the cell’s cytoskeleton of chiral filaments seems always to be involved, usually in collective states driven by polymerization forces or molecular motors. It seems particularly easy for handedness to emerge in a shear or rotation in the background of an effectively two-dimensional system, such as the cell membrane or a layer of cells, as this requires no pre-existing axis apart from the layer normal. I detail a scenario involving actin/myosin layers in snails and in C. elegans, and also one about the microtubule layer in plant cells. I also survey the other examples that I am aware of, such as the emergence of handedness in neurons, in eukaryote cell motility, and in non-flagellated bacteria.






Similar content being viewed by others
Notes
A “phage capsid” is the protein container of a virus that infects bacteria.
I am grateful to Victor Luria for this suggestion.
References
Brown, N.A., Wolpert, L.: The development of handedness in left/right asymmetry. Development 109, 1–9 (1990)
Wood, W.B.: Left-right asymmetry in animal development. Annu. Rev. Cell Dev. Biol. 13, 53–82 (1997)
Levin, M.: Left-right asymmetry in embryonic development: a comprehensive review. Mech. Dev. 122, 3–25 (2005)
Wood, W.B.: The left-right polarity puzzle: determining embryonic handedness. PLoS Biol. 3, e292 (2005)
Palmer, A.R.: Symmetry breaking and the evolution of development. Science 306, 828–833 (2004)
Henley, C.L.: Possible mechanisms for initiating macroscopic left-right asymmetry in developing organisms. In: Lebedev, V., Feigel’man, M. (eds.) Advances in Theoretical Physics, Proc. Landau 100 Memorial Conf., Chernogolovka, June 2008. AIP Conf. Proc., vol. 1134. pp. 54–61 (2009)
Frank, F.C.: On spontaneous asymmetric synthesis. Biochim. Biophys. Acta 11, 459–464 (1953)
Avertisov, V.A., Goldanskii, V.I., Kuz’min, V.A.: Handedness, origin of life, and evolution. Phys. Today 44, 33–41 (1991)
Cooke, J.: Developmental mechanism and evolutionary origin of vertebrate left/right asymmetries. Biol. Rev. 79, 377–407 (2004)
Hirokawa, N., Tanaka, Y., Okada, Y., Takeda, S.: Nodal flow and the generation of left-right asymmetry. Cell 125, 33–45 (2006)
McManus, C.: Right Hand, Left Hand: The Origins of Asymmetry in Brains, Bodies Atoms, and Cultures. Harvard University Press, Cambridge (2002), and references therein
Nonaka, S., Tanaka, Y., Okada, Y., Takeda, S., Harada, A., Kanai, Y., Kido, M., Hirokawa, N.: Randomization of left–right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 95, 829–837 (1998)
Hamada, H., Meno, C., Watanabe, D., Saijoh, Y.: Establishment of vertebrate left-right asymmetry. Nat. Rev. Genet. 3, 103–113 (2002)
Cartwright, J.H.E., Piro, O., Tuval, I.: Fluid-dynamical basis of the embryonic development of left-right asymmetry in vertebrates. Proc. Natl. Acad. Sci. USA 101, 7234–7239 (2004)
Nonaka, S., Shiratori, H., Saijoh, Y., Hamada, H.: Determination of left-right patterning of the mouse embryo by artificial nodal flow. Nature 418, 96–99 (2002)
Levin, M.: The embryonic origins of left-right asymmetry. Crit. Rev. Oral Biol. Med. 15, 197–206 (2004)
Adams, D.S., Robinson, K.R., Fukumoto, T., Yuan, S., Albertson, R.C., Yelick, P., Kuo, L., McSweeney, M., Levin, M.: Early, H+-V-ATPase-dependent proton flux is necessary for consistent left-right patterning of non-mammalian vertebrates. Development 133, 1657–1671 (2006)
Aw, S., Adams, D.S., Qiu, D., Levin, M.: H, K-ATPase protein localization and Kir4.1 function reveal concordance of three axes during early determination of left-right asymmetry. Mech. Dev. 125, 353–372 (2008).
Wood, W.B.: Evidence of handedness in C. elegans embryos for early cell interactions determining cell fates. Nature 349, 536–538 (1991)
Poole, R.J., Hobert, O.: Early embryonic patterning of neuronal left/right asymmetry in C. elegans. Curr. Biol. 16, 2279–2292 (2006)
Sun, T., Walsh, C.A.: Molecular approaches to brain asymmetry and handedness. Nature Rev., Neurosci. 7, 655 (2006)
Malashichev, Y.B., Wassersug, R.J.: Left and right in the amphibian world: which way to develop and where to turn? BioEssays 26, 512–522 (2004).
Malashichev, Ye.B., Deckel, A.W. (eds.) Behavioral and Morphological Asymmetries in Vertebrates. Landes Bioscience, Georgetown (2006)
Halpern, M.E., Liang, J.O., Gamse, J.T.: Leaning to the left: laterality in the zebrafish forebrain. Trends Neurosci. 26, 308–313 (2003).
Kawamura, R., Kakugo, A., Shikinaka, K., Osada, Y., Gong, J.P.: Ring-shaped assembly of microtubules shows preferential counterclockwise motion. Biomacromolecules 9, 2277 (2008)
Grason, G.M., Bruinsma, R.F.: Chirality and equilibrium biopolymer bundles. Phys. Rev. Lett. 99, 098101 (2007) [4 pp.]
Okumura, T., Utsuno, H., Kuroda, J., Gittenberger, E., Asami, T., Matsuno, K.: The development and evolution of left-right asymmetry in invertebrates: lessons from Drosophila and snails. Dev. Dyn. 237, 3497–3515 (2008)
Peskin, C.S., Odell, G.M., Oster, G.F.: Cellular motions and thermal fluctuations: the Brownian ratchet. Biophys. J. 65, 316–324 (1993)
Shibazaki, Y., Shimizu, M., Kuroda, R.: Body handedness is directed by genetically determined cytoskeletal dynamics in the early embryo. Curr. Biol. 14, 1462–1467 (2004)
Kuroda, R., Endo, B., Abe, M., Shimizu, M.: Chiral blastomere arrangement dictates zygotic left-right asymmetry pathway in snails. Nature 462, 790–794 (2009)
Pohl, C., Bao, Z., Pohl, C., Bao, Z.: Chiral forces organize left-right patterning in C. elegans by uncoupling midline and anteroposterior axis. Dev. Cell 19, 402–412 (2010)
Manning, M.L., Foty, R.A., Steinberg, M.S., Schoetz, E.-M.: Coaction of intercellular adhesion and cortical tension specifies tissue surface tension. Proc. Natl. Acad. Sci. USA 107, 12517 (2010)
Mabuchi, I.: Cleavage furrow: timing of emergence of contractile ring filaments and establishment of the contractile ring by filament bundling in sea urchin eggs. J. Cell Sci. 107, 1853–1862 (1994)
Kamasaki, T., Osumo, M., Mabuchi, I.: Three-dimensional arrangement of F-actin in the contractile ring of fission yeast. J. Cell Biol. 178, 765–771 (2007)
Salbreux, G., Prost, J., Joanny, J.F.: Hydrodynamics of cellular cortical flows and the formation of contractile rings, Phys. Rev. Lett. 103, 058102 (2009)
Åström, J.A., von Alfthau, S., Sunil Kumar, P.B., Karttunen, M.: Myosin motor mediated contraction is enough to produce cytokinesis in the absence of polymerisation. Soft Matter 6, 5375–5381 (2010)
Gönczy, P., Rose, L.S.: Asymmetric cell division and axis formation in the embryo, movie 3. In: WormBook. http://wormbook.org/chapters/www_asymcelldiv/asymcelldiv.html
Danilchik, M.V., Brown, E.E., Riegert, K.: Intrinsic chiral properties of the xenopus egg cortex: an early indicator of left-right asymmetry? Development 133, 4517–4526 (2006)
Fürthauer, S., Strempel, M., Grill, S.W., Jülicher, F.: Generic theory of active chiral fluids. In preparation
Strempel, M., Fürthauer, S., Grill, S.W., Jülicher, F.: Thin films of chiral motors. arXiv:1112.3492
Mayer, M., Depken, M., Bois, J., Jülicher, F., Grill, S.W.: Anisotropies in cortical tension reveal the physical basis of polarizing cortical flows. Nature 467, 617–621 (2010)
Beausang, J.F., Schroeder, H.W., Nelson, P.C., Goldman, Y.E.: Twirling of actin by myosins II and V observed via polarized TIRF in a modified gliding assay. Biophys. J. 95, 5820–5831 (2008)
Nishizaka, T., Yagi, T., Tanaka, Y., Ishiwata, S.: Right-handed rotation of an actin filament in an in vitro motile system. Nature 361, 269–271 (1993)
Vilfan, A.: Twirling motion of actin filaments in gliding assays with nonprocessive myosin motors. Biophys. J. 97, 1130 (2009)
Cowan, C.R., Hyman, A.A.: Acto-myosin reorganization and PAR polarity in C. elegans. Development 134, 1035–1043 (2007)
Ali, M.Y.: Myosin V is a left-handed spiral motor on the right-handed actin helix. Nat. Struct. Biol. 9, 464 (2002)
Morone, N., Fujiwara, T., Murase, K., Kasai, R.S., Ike, H., Yuasa, S., Usukura, J., Kusumi, A.: Three-dimensional reconstruction of the membrane skeleton at the plasma membrane interface by electron tomography. J. Cell Biol. 174, 851–862 (2006)
Vavylonis, D., Wu, J.-Q., Hao, S., O’Shaughnessy, B., Pollard, T.D.: Assembly mechanism of the contractile ring for cytokinesis by fission yeast. Science 319, 97–100 (2008)
Ziebert, F., Aranson, I.S.: Rheological and structural properties of dilute active filament solutions. Phys. Rev. E 77, 011918 (2008) [5 pp.]
Nakadera, Y., Sutcharit, C., Ubukata, T., Seki, K., Utsuno, H., Panha, S., Asami, T.: Enantiomorphs differ in shape in opposite directions between populations. J. Evol. Biol. 23, 2377–2384 (2010).
Hashimoto, T., Kato, T.: Cortical control of plant microtubules. Curr. Opin. Plant Biol. 9, 5–11 (2006).
Thitamadee, S., Tuchihara, K., Hashimoto, T.: Microtubule basis for left-handed helical growth in Arabidopsis. Nature 417, 193 (2002)
Ishida, T., Kaneko, Y., Iwano, M., Hashimoto, T.: Helical microtubule arrays in a collection of twisting tubulin mutants of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 104, 8544–8549 (2007)
Silverberg, J.L., Noar, R.D., Packer, M.S., Harrison, M.J., Henley, C.L., Cohen, I., Gerbode, S.J.: Unpublished
Thompson, M.V., Holbrook, N.M.: Root-gel interactions and the root waving behavior of Arabidopsis. Plant Pathol. 135, 1822–1837 (2004)
Hashimoto, T.: Personal communication
Wold, M.P., Gamow, R.I.: Fiber-composite model for helical growth in the Phycomyces cell wall. J. Theor. Biol. 159, 39–51 (1992)
Dixit, R., Cyr, R.: Encounters between dynamic cortical microtubules promote ordering of the cortical array through angle-dependent modifications of microtubule behavior. Plant Cell 16, 3274–3350 (2004)
Ehrhardt, D.W.: Straighten up and fly right-microtubule dynamics and organization of non-centrosomal arrays in higher plants. Curr. Opin. Cell Biol. 20, 107–116 (2008)
Chan, J., Sambade, A., Calder, G., Lloyd, C.: Arabidopsis cortical microtubules are initiated along, as well as branching from, existing microtubules. Plant Cell 21, 2298–2306 (2009)
Shaw, S.L., Kamyar, R., Ehrhardt, D.W.: Sustained microtubule treadmilling in Arabidopsis cortical arrays. Science 300, 1715–1718 (2003)
Kawamura, E., Wasteneys, G.O.: MOR1, the Arabidopsis thaliana homologue of Xenopus MAP215, promotes rapid growth and shrinkage, and suppresses the pausing of microtubules in vivo. J. Cell Sci. 121, 4114 (2008)
Baulin, V.A., Marques, C.M., Thalmann, F.: Collision induced spatial organization of microtubules. Biophys. Chem. 128, 231–244 (2007)
Hawkins, R.J., Tindemans, S.H., Mulder, B.M.: Model for the orientational ordering of the plant microtubule cortical array. Phys. Rev. E 82, 011911 (2010)
Tindemans, S.H., Hawkins, R.J., Mulder, B.M.: Survival of the aligned: ordering of the plant cortical microtubule array. Phys. Rev. Lett. 104, 058103 (2010)
Allard, J.F., Wasteneys, G.O., Cytrynbaum, E.N.: Mechanisms of self-organization of cortical microtubules in plants revealed by computational simulations. Mol. Biol. Cell 21, 278–286 (2010)
Shi, X.-Q., Ma, Y.-Q.: Understanding phase behavior of plant cell cortex microtubule organization. Proc. Natl. Acad. Sci. USA 107, 11709–11714 (2010)
Eren, E.C., Dixit, R., Gautam, N.: A three-dimensional computer simulation model reveals the mechanisms for self-organization of plant cortical microtubules into oblique arrays. Mol. Biol. Cell 21, 2674–2684 (2011)
Deinum, E.E., Tindemans, S.H., Mulder, B.M.: Taking directions: the role of microtubule-bound nucleation in the self-organization of the plant cortical array. Phys. Biol. 8, 056002 (2011)
Eren, E.C., Gautam, N., Dixit, R.: Computer simulation and mathematical models of the noncentrosomal plant cortical microtubule cytoskeleton. Cytoskeleton 69, 144–154 (2012)
Chan, J., Calder, G., Fox, S., Lloyd, C.: Cortical microtubule arrays undergo rotary movements in Arabidopsis hypocotyl epidermal cells. Nat. Cell Biol. 9, 171 (2007)
Ambrose, C., Allard, J.F., Cytrynbaum, E.N., Wasteneys, G.O.: Nat. Commun. 2, 430 (2011) [12 pp.]
Wasteneys, G.O., Ambrose, J.C.: Spatial organization of plant cortical microtubules: close encounters of the 2D kind. Trends Cell Biol. 19, 62–71 (2009)
Preston, R.D.: The case for multinet growth in growing walls of plant cells. Planta 155, 356–363 (1982)
Shen, J.X., Henley, C.L.: Unpublished
Allard, J.F., Ambrose, J.C., Wasteneys, G.O., Cytrynbaum, E.N.: A mechanochemical model explains interactions between cortical microtubules in plants. Biophys. J. 99, 1082–1090 (2010)
Wiggins, C.H., Goldstein, R.E.: Flexive and propulsive dynamics of elastica at low Reynolds number. Phys. Rev. Lett. 80, 3879 (1998).
Levin, M.: Is the early left-right axis like a plant, a kidney, or a neuron? The integration of physiological signals in embyronic asymmetry. Birth Defects Research Part C 78, 191–223 (2006)
Heacock, A.M., Agranoff, B.W.: Clockwise growth of neurites from retinal explants. Science 198, 64–66 (1977)
Tamada, A., Kawase, S., Murakami, F., Kamiguchi, H.: Autonomous right-screw rotation of growth cone filipodia drives neurite turning. J. Cell Biol. 188, 429–441 (2011)
Romijn, H.F., Mud, M.T., Wolters, P.S., Corner, M.A.: Neurite formation in dissociated cerebral cortex in vitro: evidence for clockwise outgrowth and autotopic contacts. Brain Res. 192, 575–580 (1980)
Hagmann, J., Dagan, D., Matus, A.I., Levitan, I.B.: Directional control of neurite outgrowth from cultured hippocampal neurons is modulated by the lectin concanavalin A. J. Neurobiol. 23, 354–363 (1992)
Sanjana, N.E.: Quantitative analysis of axon elongation: a novel application of stochastic modeling techniques to long-term, high-resolution time-lapse microscopy of cortical neurons, PhD thesis. MIT (2010). URL: http://dspace.mit.edu/bitstream/handle/1721.1/58281/639302908.pdf
Lionnet, T., Joubaud, S., Lavery, R., Bensimon, D., Croquette, V.: Wringing out DNA. Phys. Rev. Lett. 96, 178102 (2006)
Gore, J., Bryant, Z., Nöllmann, M., Le, M.U., Cozzarelli, N.R., Bustamante, C.: DNA overwinds when stretched. Nature 442, 836 (2006)
Kamien, R.D., Lubensky, T.C., Nelson, P., O’Hern, C.S.: Direct determination of DNA twist-stretch coupling. Europhys. Lett. 38, 237–242 (1997).
Arrieumerlou, C., Meyer, T.: A local coupling model and compass parameter for eukaryotic chemotaxis. Dev. Cell 8, 215–227 (2005)
Xu, J., van Kaymeulen, A., Wakida, N.M., Carlton, P., Berns, M.W., Bourne, H.R.: Polarity reveals intrinsic cell chirality. Proc. Natl. Acad. Sci. USA 104, 9296–9300 (2007)
Chuan, T.-H., Hsu, J.J., Zhao, X., Guo, C., Wong, M.N., Huang, Y., Li, Z., Garfinkel, A., Ho, C.-M., Tintut, Y., Demer, L.L.: Left-right symmetry breaking in tissue morphogenesis via cytoskeletal mechanics. Circ. Res. 110, 551–559 (2012)
Wan, L.Q., Ronaldson, K., Park, M., Taylor, G., Zhang, Y., Gimble, J.M., Vunjak-Novakovic, G.: Micropatterned mammalian cells exhibit phenotype-specific left-right asymmetry. Proc. Natl. Acad. Sci. USA 108, 12295–12300 (2011)
Spéder, P., Adam, G., Noselli, S.: Type ID unconventional myosin controls left-right asymmetry in Drosophila. Nature 440, 803–807 (2006)
Hozumi, S., Maeda, R., Taniguchi, K., et al.: An unconventional myosin in Drosophila reverses the default handedness in visceral organs. Nature 440, 798–802 (2006)
Spéder, P., Noselli, S.: Left-right asymmetry: class I myosins show the direction. Curr. Opin. Cell Biol. 19, 82–87 (2007)
Taniguchi, K., Maeda, R., Ando, T., Okumura, T., Nakazawa, N., Hatori, R., Nakamura, M., Hozumi, S., Fujiwara, H., Matsuno, K.: Chirality in planar cell shape contributes to left-right asymmetric epithelial morphogenesis. Science 333, 339 (2011)
Lauga, W., DiLuzio, W.R., Whitesides, G.M., Stone, H.A.: Swimming in circles: motion of bacteria near solid boundaries. Biophys. J. 90, 400–412 (2006)
DiLuzio, W.R., Turner, L., Mayer, M., Garstecki, P., Weibel, D.B., Berg, H.C., Whitesides, G.M.: Escherichia coli swim on the right-hand side. Nature 435, 1271–1274 (2005)
Wu, Y., Hosu, B.G., Berg, H.C.: Microbubbles reveal chiral fluid flows in bacterial swarms. Proc. Natl. Acad. Sci. USA 108, 4147–4151 (2011)
Ben-Jacob, E., Cohen, I., Shochet, O., Tenenbaum, A., Czirók, A., Vicsek, T.: Cooperative formation of chiral patterns during growth of bacterial colonies, Phys. Rev. Lett. 75, 2899 (1995)
Ben-Jacob, E., Schochet, O., Tenenbaum, A., Cohen, I., Csirok, A., Vicsek, T.: Generic modelling of cooperative growth patterns in bacterial colonies. Nature 368, 46–49 (2004)
Levine, H., Ben-Jacob, E., Cohen, E., Rappel, W.-J.: In: Proc. 45th IEEE Conference on Decision and Control, p. 5073 (2006)
Mendelson, N.H.: Helical growth of Bacillus subtilis: a new model of cell growth. Proc. Natl. Acad. Sci. USA 73, 1740–1744 (1976)
Varma, A., de Pedro, M.A., Young, K.D.: FtsZ directs a second mode of peptidoglycan synthesis in Escherichia coli. J. Bacteriol. 189, 5692–5704 (2007)
Jones, L.F., Carballido-Lopez, R., Errington, J.: Cell 104, 913 (2001)
Wang, S., Furchtgott, L., Huang, K.C., Shaevitz, J.W.: Helical insertion of peptidoglycan produces chiral ordering of the bacterial cell wall. Proc. Natl. Acad. Sci. USA 109, E595–E604 (2012)
Figge, R.M., Divakaruni, A.V., Gober, J.W.: MreB, the cell shape-determining bacterial actin homologue, co-ordinates cell wall morphogenesis in Caulobacter crescentus. Mol. Microbiol. 51, 1321–1332 (2004)
Wolgemuth, C.W., Inclan, Y.F., Quan, J., Mukherjee, S., Oster, G., Koehl, M.A.: How to make a spiral bacterium. Phys. Biol. 2, 189–199 (2005)
Mendelson, N.H., Sarlls, J.E., Wolgemuth, C.W., Goldstein, R.E.: Chiral self-propulsion of growing bacterial macrofibers on a solid surface. Phys. Rev. Lett. 84, 1627–1630 (2000)
Mendelson, N.H., Thwaites, J.J., Kessler, J.O. Li, C.: Mec (1995)
Andrews, S.S., Arkin, A.P.: A mechanical explanation for cytoskeletal rings and helices in bacteria. Biophys. J. 93, 1872–1884 (2007)
Salje, J., van den Ent, F., de Boer, P., Löwe, J.: Direct membrane binding by bacterial actin MreB. Mol. Cell 43, 478–487 (2011)
Acknowledgements
I thank S. Grill, T. Hashimoto, F. Jülicher, M. Levin, V. Luria, N. Sanjana, S. Seung, E.D. Siggia, G.O. Wasteneys, N. Wingreen, R. Chachra, and J.X. Shen, for discussions and communications (and the last two for collaborations). This work was supported by the U.S. Dept. of Energy, grant DE-FG-ER45405.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Henley, C.L. Possible Origins of Macroscopic Left-Right Asymmetry in Organisms. J Stat Phys 148, 741–775 (2012). https://doi.org/10.1007/s10955-012-0520-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10955-012-0520-z