Abstract
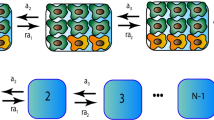
We study the stochastic dynamics of the two most common patterns in cancer initiation and progression: loss-of-function and gain-of-function mutations. We consider three stochastic models of cell populations with a constant size: a mass-action model, a spatial model and a hierarchical model. For gain-of-function mutations, we calculate the probability of mutant fixation starting from one mutant cell. For loss-of-function mutations, we calculate the rate of production of double-hit mutants. It turns out that the results are different in all models. This suggests that simple mass-action models are often misleading when studying cancer dynamics. Moreover, our results also allow us to think about various types of tissue architecture and its protective role against cancer. In particular, we show that hierarchical tissue organization lowers the risk of cancerous transformations. Also, cellular motility and long-range signaling can decrease the risk of cancer in solid tissues.
Similar content being viewed by others
References
1. R. Araujo and D. McElwain, A history of the study of solid tumor growth: The contribution of mathematical modeling. Bull. Math Biol. 66(5):1039–1091 (2004).
2. N. Bellomo, A. Bellouquid, and M. Delitala, Mathematical topics in the modeling complex multicellular systems and tumor immune cells competition. Math. Mod. Meth. Appl. Sci. 14:1683–1733 (2004).
3. N. Bellomo and L. Preziosi, Modeling and mathematical problems related to tumor evolution and its interation with the immune system. Math. Comp. Modeling 22(3–4):413–452 (2000).
4. A. Bellouquid and B. Delitala, Mathematical methods and tools of kinetic theory towards modelling complex biological systems. Math. Mod. Meth. Appl. Sci. 15:1639–1666 (2005).
5. M. Bjerknes, Expansion of mutant stem cell populations in the human colon. J. Theor. Biol. 178(4):381–385 (1996).
6. J. Cairns, Mutation selection and the natural history of cancer. Nature 255(5505):197–200 (1975).
7. M. Chaplain, Avascular growth, angiogenesis and vascular growth in solid tumors. Math. Comp. Modeling 23(6):47–87 (1996).
8. M. Chaplain and G. Lolas, Spatio-temporal heterogeneity arising in a mathematical model of cancer invasion of tissue. Math. Mod. Meth. Appl. Sci. 15:1685–1734 (2005).
9. A. Dewanji, S. H. Moolgavkar, and E. G. Luebeck, Two-mutation model for carcinogenesis: joint analysis of premalignant and malignant lesions. Math. Biosci. 104(1):97–109 (1991).
10. W. D. Hazelton, M, S. Clements, and S. H. Moolgavkar, Multistage carcinogenesis and lung cancer mortality in three cohorts. Cancer Epidemiol. Biomarkers Prev. 14(5):1171–1181 (2005).
11. W. F. Heidenreich, E. G. Luebeck, W. D. Hazelton, H. G. Paretzke, and S. H. Moolgavkar, Multistage models and the incidence of cancer in the cohort of atomic bomb survivors. Radiat. Res. 158(5):607–614 (2002).
12. W. F. Heidenreich, E. G. Luebeck, and S. H. Moolgavkar, Some properties of the hazard function of the two-mutation clonal expansion model. Risk Anal. 17(3):391–399 (1997).
13. N. Heisterkamp, K. Stam, J. Groffen, A. de Klein, and G. Grosveld, Structural organization of the bcr gene and its role in the Ph' translocation. Nature 315(6022):758–761 (1985).
14. Y. Iwasa, F. Michor and M. A. Nowak, Stochastic tunnels in evolutionary dynamics. Genetics 166(3):1571–1579 (2004).
15. S. Karlin and H. Taylor, A First Course in Stochastic Processes, Second Edition (Academic Press, New York, 1975).
16. A. G. J. Knudson, Mutation and cancer: statistical study of retinoblastoma. Proc. Natl. Acad. Sci. USA 68(4):820–823 (1971).
17. N. Komarova, Spatial stochastic models for cancer initiation and progression. Bull. Math. Biol., DOI: 10.1007/s11538-005-9046-8:1–27 (2006).
18. N. Komarova and P. Cheng, Epithelial tissue architecture protects against cancer. Math. Biosci. 200(1):90–117 (2006).
19. N. L. Komarova, A. Sengupta, and M. A. Nowak, Mutation-selection networks of cancer initiation: tumor suppressor genes and chromosomal instability. J. Theor. Biol. 223(4):433–450 (2003).
20. N. L. Komarova and L. Wang, Initiation of colorectal cancer: Where do the two hits hit? Cell Cycle 3(12):1558–1565 (2004).
21. N. L. Komarova and D. Wodarz, The optimal rate of chromosome loss for the inactivation of tumor suppressor genes in cancer. Proc. Natl. Acad. Sci. USA 101(18):7017–7021 (2004).
22. C. Lengauer, K. W. Kinzler, and B. Vogelstein, Genetic instabilities in human cancers. Nature 396(6712):643–649 (1998).
23. M. P. Little and E. G. Wright, A stochastic carcinogenesis model incorporating genomic instability fitted to colon cancer data. Math. Biosci. 183(2):111–134 (2003).
24. L. A. Loeb, Mutator phenotype may be required for multistage carcinogenesis. Cancer Res. 51(12):3075–3079 (1991).
25. L. A. Loeb, A mutator phenotype in cancer. Cancer Res. 61(8):3230–3239 (2001).
26. E. G. Luebeck, W. F. Heidenreich, W. D. Hazelton, H. G. Paretzke, and S. H. Moolgavkar. Biologically based analysis of the data for the Colorado uranium miners cohort: age, dose and dose-rate effects. Radiat. Res. 152(4):339–351 (1999).
27. E. G. Luebeck and S. H. Moolgavkar, Multistage carcinogenesis and the incidence of colorectal cancer. Proc. Natl. Acad. Sci. USA 99(23):15095–15100 (2002).
28. F. Michor, Y. Iwasa, H. Rajagopalan, C. Lengauer, and M. A. Nowak, Linear model of colon cancer initiation. Cell Cycle 3(3):358–362 (2004).
29. S. H. Moolgavkar, The multistage theory of carcinogenesis and the age distribution of cancer in man. J. Natl. Cancer Inst. 61(1):49–52 (1978).
30. M. A. Nowak, F. Michor, N. L. Komarova, and Y. Iwasa, Evolutionary dynamics of tumor suppressor gene inactivation. Proc. Natl. Acad. Sci. USA 101(29):10635–10638 (2004).
31. E. Parzen, Stochastic Processes, vol. 24 of Classics in Applied Mathematics (SIAM, 1999).
32. S. Ro and B. Rannala, Methylation patterns and mathematical models reveal dynamics of stem cell turnover in the human colon. Proc. Natl. Acad. Sci. USA 98(19):10519–10521 (2001). Comment.
33. T. Strachan and A. P. Read, Human Molecular Genetics 2 (John Wiley & Sons, Inc., 1999).
34. W. Y. Tan, Stochastic Models of Carcinogenesis (Marcel Drekker, New York, 1991).
35. W. Y. Tan and C. W. Chen, Stochastic models of carcinogenesis, some new insight. Math. Comput. Modeling 28:49–71 (1998).
36. B. Vogelstein and K. W. Kinzler, The Genetic Basis of Human Cancer (McGraw-Hill, 1997).
37. B. Vogelstein and K. W. Kinzler, Cancer genes and the pathways they control. Nat. Med. 10(8):789–799 (2004).
38. D. Wodarz and N. Kornarova, Computational Biology of Cancer: Lecture Notes and Mathematical Modeling (World Scientific, 2005).
39. Y. Yatabe, S. Tavare, and D. Shibata, Investigating stem cells in human colon by using methylation patterns. Proc. Natl. Acad. Sci. USA 98(19):10839–10844 (2001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Komarova, N.L. Loss- and Gain-of-Function Mutations in Cancer: Mass-action, Spatial and Hierarchical Models. J Stat Phys 128, 413–446 (2007). https://doi.org/10.1007/s10955-006-9238-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10955-006-9238-0