Abstract
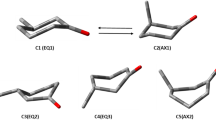
Density Functional Theory (DFT) calculations of infrared spectra for the optimized geometries of the R-(+)-3-methylcyclopentanone (R3MCP) equatorial-methyl and axial-methyl conformers were performed in eleven common solvents with a wide polarity range, within the framework of polarizable continuum model (PCM). The DFT correlation function type B3LYP, using a powerful basis set (aug-cc-pVDZ), yielded different linear correlations between solvent polarity and R3MCP equatorial and axial conformers for frequencies of IR modes, intensities, and enthalpies. DFT calculations of the dipole moments of R3MCP equatorial and axial conformer components in 3D were also carried out and found to have a linear correlation with the solvent polarity. An increasing trend for the hypsochromic (blue) shift in the equatorial conformer’s IR frequencies is observed, in comparison to bathochromic (red) shift for the axial-methyl conformer IR modes, as a function of the solvent polarity.





Similar content being viewed by others
References
Richardson, F.S., Shillady, D.D., Bloor, J.E.: The optical activity of alkyl-substituted cyclopentanones. INDO molecular orbital model. J. Phys. Chem. 75, 2466–2479 (1971)
Flament, J.P., Gervais, H.P.: Optical-activity of 3-methyl-cyclopentanone—ab initio calculation. Tetrahedron 36, 1949–1952 (1980)
Li, Y.S.: Microwave-spectrum, dipole-moment, and conformation of 3-methylcyclopentanone. J. Mol. Spectrosc. 104, 302–307 (1984)
Potts, A.R., Nesselrodt, D.R., Baer, T., Driscoll, J.W., Bays, J.P.: The 3s Rydberg spectra and conformations of methyl-substituted cyclopentanones. J. Phys. Chem. 99, 12090–12098 (1995)
He, J., Petrovic, A.G., Polavarapu, P.L.: Determining the conformer populations of (R)-(+)-3-methylcyclopentanone using vibrational absorption, vibrational circular dichroism, and specific rotation. J. Phys. Chem., B 108, 20451–20457 (2004)
Li, R., Sullivan, R., Al-Basheer, W., Pagni, R.M., Compton, R.N.: Linear and nonlinear circular dichroism of R-(+)-3-methylcyclopentanone. J. Chem. Phys. 125, 144304 (2006)
Al-Basheer, W., Pagni, R.M., Compton, R.N.: Spectroscopic and theoretical investigation of (R)-3-methylcyclopentanone. The effect of solvent and temperature on the distribution of conformers. J. Phys. Chem., A 111, 2293–2298 (2007)
Kim, D., Baer, T.: Gas-phase measurement of ΔH ∘ between axial and equatorial conformations of 3-methylcyclopentanone. Chem. Phys. 256, 251–258 (2000)
Lee, C., Yang, W., Parr, R.G.: Development of the Colle–Salvetti correlation-energy formula into a functional of the electron-density. Phys. Rev., B 37, 785–789 (1988)
Parr, R.G., Yang, W.: Density Functional Theory of Atoms and Molecules. Oxford University Press, New York (1989)
Becke, A.D.: Density functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993)
Onsager, L.: Electric moments of molecules in liquids. J. Am. Chem. Soc. 58, 1486–1493 (1936)
Bauer, E., Magat, M.: Regarding the deformation of molecules in a condensed phase, and the “hydrogen bond”. J. Phys. Radium 9, 319–330 (1938)
Hirota, E.: The electrostatic effect of the solvents on the frequency shifts and the intensity changes of the infrared absorption spectra. Bull. Chem. Soc. Jpn. 27, 295–297 (1954)
Mallard, W.C., Straley, J.W.: Vibrational intensities in halogenated methanes. III. The interpretation of solution data. J. Chem. Phys. 27, 877–879 (1957)
Person, W.B.: Liquid–gas infrared intensities, pressure-induced absorption, and the temperature dependence of infrared intensities in liquids. J. Chem. Phys. 28, 319–322 (1958)
Buckingham, A.D.: Solvent effects in infra-red spectroscopy. Proc. R. Soc. Lond. Ser. A, Math. Phys. Sci. 248, 169–182 (1958)
Buckingham, A.D.: A theory of frequency, intensity and band-width changes due to solvents in infra-red spectroscopy. Proc. R. Soc. Lond. Ser. A, Math. Phys. Sci. 255, 32–39 (1960)
Mirone, P.: Solvent effects on Raman and infra-red intensities in continuous dielectric model. Spectrochim. Acta 22, 1897–1905 (1966)
Miertus, S., Scrocco, E., Tomasi, J.: Electrostatic interaction of a solute with a continuum. A direct utilization of ab initio molecular potentials for the prevision of the solvent effects. Chem. Phys. 55, 117–129 (1981)
Cammi, R., Cappelli, C., Corni, S., Tomasi, J.: On the calculation of infrared intensities in solution within the polarizable continuum model. J. Phys. Chem., A 104, 9874–9879 (2000)
Rizzo, A., Lin, N., Ruud, K.: Ab initio study of the one- and two-photon circular dichroism of R-(+)-3-methylcyclopentanone. J. Chem. Phys. 128, 164312 (2008)
Pipolo, S., Cammi, R., Rizzo, A., Cappelli, C., Mennucci, B., Tomasi, J.: Cavity field effects within a polarizable continuum model of solvation: application to the calculation of electronic circular dichroism spectra of R-(+)-3-methylcyclopentanone. Int. J. Quant. Chem. 111, 826–838 (2011)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Montgomery, J.A., Jr., Vreven, T., Kudin, K.N., Burant, J.C., Millam, J.M., Iyengar, S.S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G.A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J.E., Hratchian, H.P., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Ayala, P.Y., Morokuma, K., Voth, G.A., Salvador, P., Dannenberg, J.J., Zakrzewski, V.G., Dapprich, S., Daniels, A.D., Strain, M.C., Farkas, O., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Ortiz, J.V., Cui, Q., Baboul, A.G., Clifford, S., Cioslowski, J., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Gonzalez, C., Pople, J.A.: Gaussian 03, Revision B.03. Gaussian, Inc., Pittsburgh (2003)
Scott, A.P., Radom, L.: Harmonic vibrational frequencies: an evaluation of Hartree–Fock, Moller–Plesset, quadratic configuration interaction, density functional theory, and semiempirical scale factors. J. Phys. Chem. 100, 16502–16513 (1996)
Miertus, S., Tomasi, J.: Approximate evaluations of the electrostatic free-energy and internal energy changes in solution processes. Chem. Phys. 65, 239–245 (1982)
Cossi, M., Barone, V., Cammi, R., Tomasi, J.: Ab initio study of solvated molecules: A new implementation of the polarizable continuum model. Chem. Phys. Lett. 255, 327–335 (1996)
Dimroth, K., Reichardt, C., Siepmann, T., Bohlmann, F.: Uber Pyridinium-n-phenol betaine und ihre verwendung zur charakterisierung der polaritat von losungsmitteln. Liebigs Ann. Chem. 661, 1–37 (1963)
Kamlet, M.J., Abboud, J.L., Taft, R.W.: Solvatochromic comparison method. 6. π ∗ of solvent polarities. J. Am. Chem. Soc. 99, 6027–6038 (1977)
Reichardt, C.: Solvatochromism, thermochromism, piezochromism, halochromism, and chiro-solvatochromism of pyridinium N-phenoxide betaine dyes. Chem. Soc. Rev. 21, 147–153 (1992)
Reichardt, C.: Solvents and Solvent Effects in Organic Chemistry, 3rd edn. Wiley-VCH, Weinheim (2003)
Marcus, Y.: The Properties of Solvents. Wiley, Chichester (1998)
Acknowledgements
This work is dedicated to the memory of Mississippi State University physics graduate student Shereen Shawaqfeh whose life ended prematurely in a tragic car accident on September 18, 2010. Shereen was a friend, a fine scholar, and a colleague at the Hashemite University in Jordan. Her enthusiasm, dedication, and commitment to pursue a career in science continues to inspire many women.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Al-Basheer, W. Solvent Effects on IR Modes of (R)-3-Methylcyclopentanone Conformers: A Computational Investigation. J Solution Chem 41, 1495–1506 (2012). https://doi.org/10.1007/s10953-012-9890-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-012-9890-8