Abstract
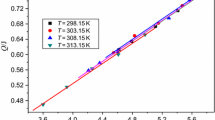
The enthalpies of dissolution of 1,2,3-triazole nitrate in water were measured using a RD496-2000 Calvet microcalorimeter at four different temperatures under atmospheric pressure. Differential enthalpies (Δdif H) and molar enthalpies (Δdiss H) of dissolution were determined. The corresponding kinetic equations that describe the dissolution rate at the four experimental temperatures are \(\frac{d\alpha}{dt} / \mathrm{s}^{ - 1} =10^{ - 3.75}( 1 - \alpha)^{0.96}\) (T=298.15 K), \(\frac{d\alpha}{dt} /\mathrm{s}^{ - 1} = 10^{ - 3.73}( 1 - \alpha)^{1.00}\) (T=303.15 K), \(\frac{d\alpha}{dt} / \mathrm{s}^{ - 1} = 10^{ - 3.72}( 1 - \alpha)^{0.98}\) (T=308.15 K) and \(\frac{d\alpha}{dt} / \mathrm{s}^{ - 1} = 10^{ - 3.71}( 1 -\alpha)^{0.97}\) (T=313.15 K). The determined values of the activation energy E and pre-exponential factor A for the dissolution process are 5.01 kJ⋅mol−1 and 10−2.87 s−1, respectively.




Similar content being viewed by others
References
Gao, H.X., Ye, C.F., Piekarski, C.M., Shreeve, T.M.: Computational characterization of energetic salts. J. Phys. Chem. C 111, 10718–10726 (2007)
Agrawal, J.P.: Recent trends in high-energy materials. Prog. Energy Combust. Sci. 24, 1–13 (1998)
Huang, H.F., Meng, Z.H., Zhou, Z.M., Gao, H.X., Zhang, J., Wu, Y.K.: Energetic salts and energetic ionic liquids. Prog. Chem. 21, 152–161 (2009) (in Chinese)
Drake, G., Kaplan, G., Hall, L., Hawkins, T., Larue, J.: A new family of energetic ionic liquids 1-amino-3-alkyl-1,2,3-triazolium nitrates. J. Chem. Crystallogr. 37, 15–22 (2007)
Ye, C.F., Shreeve, J.M.: Rapid and accurate estimation of densities of room-temperature ionic liquids and salts. J. Phys. Chem. A 111, 1456–1461 (2007)
Tong, B., Liu, Q.S., Tan, Z.C., Welz-Biermann, U.: Thermochemistry of alkyl pyridinium bromide ionic liquids: calorimetric measurements and calculation. J. Phys. Chem. A 114, 3782–3787 (2010)
Krossing, I., Slattery, J.M., Daguenet, C., Dyson, P.J., Oleinikova, A., Weingärtner, H.: Why are ionic liquids liquid? A simple explanation based on lattice and solvation energies. J. Am. Chem. Soc. 128, 13427–13434 (2006)
Kolaski, M., Lee, H.M., Pak, C., Kim, K.S.: Charge-transfer-to-solvent-driven dissolution dynamics of 1-(H2O)2–5 upon excitation: excited-state ab initio molecular dynamics simulations. J. Am. Chem. Soc. 130, 103–112 (2008)
Emel’yanenko, V.N., Verevkin, S.P., Heintz, A.: Imidazolium-based ionic liquids 1-methyl imidazolium nitrate: thermochemical measurements and ab initio calculations. J. Phys. Chem. B 113, 9871–9881 (2009)
Domanska, U., Marciniak, A.: Activity coefficients at infinite dilution measurements for organic solutes and water in the ionic liquid 1-ethyl-3-methylimidazolium trifluoroacetate. J. Phys. Chem. B 111, 11984–11988 (2007)
Fischer, G., Holl, G., Klapötke, T.M., Weigand, J.J.: A study on thermal decomposition behavior of derivatives of 1,5-diamino-1H-tetrazole (DAT): a new family of energetic heterocyclic-based salts. Thermochim. Acta 437, 168–175 (2005)
Chowdhury, A., Thynell, S.T.: Confined rapid thermolysis/FTIR/ToF studies of triazolium-based energetic ionic liquids. Thermochim. Acta 466, 1–11 (2007)
Chowdhury, A., Thynell, S.T., Lin, P.: Confined rapid thermolysis/FTIR/ToF studies of triazolium-based energetic ionic liquids. Thermochim. Acta 485, 1–12 (2009)
Ou, Y.X., Liu, J.Q.: High Energy Density Compounds. National Defense Industry Press, Beijing (2005)
Marthada, V.K.: The enthalpy of solution of SRM(KCl) in H2O. J. Res. Natl. Bur. Stand., A Phys. Chem. 85, 467–481 (1980)
Hu, R.Z., Gao, S.L., Zhao, F.Q., Shi, Q.Z., Zhang, T.L., Zhang, J.G.: Thermal Analysis Kinetics 2nd edn. Science Press, Beijing (2008) (in Chinese)
Xue, L., Zhao, F.Q., Xing, X.L., Gao, H.X., Xu, S.Y., Hu, R.Z.: Dissolution properties of 1,3,3-trinitroazetidine (TNAZ) in ethyl acetate and N,N-dimethylformamide. Acta Phys. Chim. Sin. 25, 2413–2421 (2009)
Xing, X.L., Xue, L., Zhao, F.Q., Gao, H.X., Hu, R.Z.: Thermochemical properties of 1,1-diamino-2,2-dinitroethylene (FOX-7) in dimethyl sulfoxide (DMSO). Thermochim. Acta 35, 491–497 (2009)
Xue, L., Zhao, F.Q., Hu, R.Z., Gao, H.X.: A simple method to estimate the critical temperature of thermal explosion for energetic materials using nonisothermal DSC. J. Energ. Mater. 28, 17–31 (2010)
Kolker, A.M., Safonova, L.P.: Molar heat capacities of the (water+acetonitrile) mixtures at T=(283.15,298.15,313.15, and 328.15) K. J. Chem. Thermodyn. 42, 1209–1212 (2010)
Xue, L., Zhao, F.Q., Xing, X.L., Zhou, Z.M., Wang, K., Gao, H.X., Yi, J.H., Hu, R.Z.: Dissolution of 3,4,5-triamino-1,2,4-triazole dinitramide in N-methyl pyrrolidone. J. Chin. Chem. Soc. 57, 338–342 (2010)
Rodríguez, S.J., Cristancho, D.M., Neita, P.C., Vargas, E.F., Martínez, F.: Solution thermodynamics of ethylhexyl triazone in some ethanol+ethyl acetate mixtures. J. Solution Chem. 39, 1122–1133 (2010)
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 20573098) and the Science and Technology Foundation of the National Key Lab of Science and Technology on Combustion and Explosion in China (Grant No. 9140C3501020901).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xue, L., Zhao, F., Xing, X. et al. Dissolution Thermodynamics of 1,2,3-Triazole Nitrate in Water. J Solution Chem 41, 17–24 (2012). https://doi.org/10.1007/s10953-011-9777-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-011-9777-0