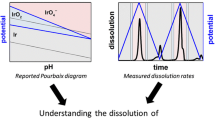
Abstract
The solubility of industrially produced titanium dioxide nanoparticles has been studied in aqueous sodium chloride media in the pH range 1 to 13 at 25 °C by using adsorptive stripping voltammetry (AdSV). Kinetic dissolution curves have been obtained as well as long-term solubilities that provide an approximation of the equilibrium solubilities. The titania nanoparticles used in the dissolution experiments have been characterized by nitrogen sorption measurements, XRD and colloid titration. The equilibrium solubilities and titanium(IV) speciation and their dependences on pH have been modelled by assuming the formation of the mononuclear titanium hydroxo complexes [Ti(OH) n ](4−n)+ (n=2 to 5) to be the only titanium species present. The solubility product of titanium dioxide and equilibrium constants for titanium(IV) hydrolysis, calculated from the AdSV solubility data, are presented.
Similar content being viewed by others
References
Grätzel, M.: Photoelectrochemical cells. Nature 414, 338–344 (2001). doi:10.1038/35104607
Zhang, Z., Wang, C., Zakaria, R., Ying, J.Y.: Role of particle size in nanocrystalline TiO2-based photocatalysts. J. Phys. Chem. B 102, 10871–10878 (1998). doi:10.1021/jp982948+
Holleman, A.F., Wiberg, N.: Lehrbuch der Anorganischen Chemie/Holleman-Wiberg. de Gruyter, Berlin/New York (1995)
Borm, P., Klaessig, F.C., Landry, T.D., Moudgil, B., Pauluhn, J., Thomas, K., Trottier, R., Wood, S.: Research strategies for safety evaluation of nanomaterials, Part V: Role of dissolution in biological fate and effects of nanoscale particles. Toxicol. Sci. 90, 23–32 (2006). doi:10.1093/toxsci/kfj084
Oberdörster, G., Oberdörster, E., Oberdörster, J.: Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ. Heal. Perspect. 113, 823–839 (2005)
Nel, A., Xia, T., Mädler, L., Li, N.: Toxic potential of materials at the nanolevel. Science 311, 622–627 (2006). doi:10.1126/science.1114397
Finnegan, M.P., Zhang, H., Banfield, J.F.: Anatase coarsening kinetics under hydrothermal conditions as a function of pH and temperature. Chem. Mater. 20, 3443–3449 (2008). doi:10.1021/cm071057o
Jolivet, J., Cassaignon, S., Chanéac, C., Chiche, D., Tronc, E.: Design of oxide nanoparticles by aqueous chemistry. J. Sol-Gel Sci. Technol. 46, 299–305 (2008). doi:10.1007/s10971-007-1645-4
Testino, A., Bellobono, I.R., Buscaglia, V., Canevali, C., D’Arienzo, M., Polizzi, S., Scotti, R., Morazzoni, F.: Optimizing the photocatalytic properties of hydrothermal TiO2 by the control of phase composition and particle morphology. A systematic approach. J. Am. Chem. Soc. 129, 3564–3575 (2007). doi:10.1021/ja067050+
Reyes-Coronado, D., Rodríguez-Gattorno, G., Espinosa-Pesqueira, M.E., Cab, C., de Coss, R., Oskam, G.: Phase-pure TiO2 nanoparticles: anatase. Brookite and rutile. Nanotechnology 19, 145605 (2008) (10 pp.)
Sugimoto, T., Zhou, X., Muramatsu, A.: Synthesis of uniform anatase TiO2 nanoparticles by gel-sol method 1. Solution chemistry of Ti(OH) (4−n)+ n complexes. J. Colloid Interface Sci. 252, 339–346 (2002). doi:10.1006/jcis.2002.8454
Pottier, A., Cassaignon, S., Chanéac, C., Villain, F., Tronc, E., Jolivet, J.: Size tailoring of TiO2 anatase nanoparticles in aqueous medium and synthesis of nanocomposites. Characterization by Raman spectroscopy. J. Mater. Chem. 13, 877–882 (2003). doi:10.1039/b211271j
Atashfaraz, M., Niassar, M.S., Ohara, S., Minami, K., Umetsu, M., Naka, T., Adschiri, T.: Effect of titanium dioxide solubility on the formation of BaTiO3 nanoparticles in supercritical water. Fluid Phase Equilib. 257, 233–237 (2007). doi:10.1016/j.fluid.2007.03.025
Schmidt, J., Vogelsberger, W.: Dissolution kinetics of titanium dioxide nanoparticles: the observation of an unusual kinetic size effect. J. Phys. Chem. B 110, 3955–3963 (2006). doi:10.1021/jp055361l
Vogelsberger, W., Schmidt, J., Roelofs, F.: Dissolution kinetics of oxidic nanoparticles: the observation of an unusual behaviour. Colloids Surf. A Physicochem. Eng. Asp. 324, 51–57 (2008). doi:10.1016/j.colsurfa.2008.03.032
Roelofs, F., Vogelsberger, W.: Dissolution kinetics of nanodispersed γ-alumina in aqueous solution at different pH: unusual kinetic size effect and formation of a new phase. J. Colloid Interface Sci. 303, 450–459 (2006). doi:10.1016/j.jcis.2006.08.016
Ziemniak, S.E., Jones, M.E., Combs, K.E.S.: Solubility behavior of titanium(IV) oxide in alkaline media at elevated temperatures. J. Solution Chem. 22, 601–623 (1993). doi:10.1007/BF00646781
Knauss, K.G., Dibley, M.J., Bourcier, W.L., Shaw, H.F.: Ti(IV) hydrolysis constants derived from rutile solubility measurements made from 100 to 300 °C. Appl. Geochem. 16, 1115–1128 (2001). doi:10.1016/S0883-2927(00)00081-0
Antignano, A., Manning, C.E.: Rutile solubility in H2O, H2O–SiO2, and H2O–NaAlSi3O8 fluids at 0.7–2.0 GPa and 700–1000 °C: implications for mobility of nominally insoluble elements. Chem. Geol. 255, 283–293 (2008). doi:10.1016/j.chemgeo.2008.07.001
Audétat, A., Keppler, H.: Solubility of rutile in subduction zone fluids, as determined by experiments in the hydrothermal diamond anvil cell. Earth Planet. Sci. Lett. 232, 393–402 (2005). doi:10.1016/j.epsl.2005.01.028
Schuiling, R.D., Vink, B.W.: Stability relations of some titanium-minerals (sphene, perovskite, rutile, anatase). Geochim. Cosmochim. Acta 31, 2399–2411 (1967). doi:10.1016/0016-7037(67)90011-7
Leturcq, G., Advocat, T., Hart, K., Berger, G., Lacombe, J., Bonnetier, A.: Solubility study of Ti,Zr-based ceramics designed to immobilize long-lived radionuclides. Am. Mineral. 86, 871–880 (2001)
Liberti, A., Chiantella, V., Corigliano, F.: Mononuclear hydrolysis of titanium(IV) from partition equilibria. J. Inorg. Nucl. Chem. 25, 415–427 (1963). doi:10.1016/0022-1902(63)80192-X
Nabivanets, B.I., Lukachina, V.V.: Hydroxy complexes of titanium(IV). Ukr. Khim. Zhur. 30, 1123–1128 (1964)
Kelsall, G.H., Robbins, D.J.: Thermodynamics of Ti–H2O–F(–Fe) Systems at 298 K. J. Electroanal. Chem. 283, 135–157 (1990). doi:10.1016/0022-0728(90)87385-W
Comba, P., Merbach, A.: The titanyl question revisited. Inorg. Chem. 26, 1315–1323 (1987). doi:10.1021/ic00255a024
Einaga, H.: Hydrolysis of titanium(IV) in aqueous (Na, H)Cl solution. Dalton Trans. 12, 1917–1919 (1979)
Yokoi, K., van den Berg, C.M.G.: Determination of titanium in sea water using catalytic cathodic stripping voltammetry. Anal. Chim. Acta 245, 167–176 (1991). doi:10.1016/S0003-2670(00)80217-2
Schmidt, J.: Charakterisierung des Löseverhaltens oxidischer Nanopartikel (TiO2, ZrO2, SiO2) in wässrigen Systemen. Thesis, Friedrich-Schiller-Universität Jena, Jena, Germany (2008)
Kraus, W., Nolze, G.: PowderCell for Windows Version 2.4. Bundesanstalt für Materialforschung und –prüfung, Berlin (2000)
Barringer, E.A., Bowen, H.K.: High-purity, monodisperse TiO2 powders by hydrolysis of titanium tetraethoxide. 1. Synthesis and physical properties. Langmuir 1, 414–420 (1985). doi:10.1021/la00064a005
Löbbus, M., Vogelsberger, W., Sonnefeld, J., Seidel, A.: Current considerations for the dissolution kinetics of solid oxides with silica. Langmuir 14, 4386–4396 (1998). doi:10.1021/la9712451
Kosmulski, M.: The significance of the difference in the point of zero charge between rutile and anatase. Adv. Colloid Interface Sci. 99, 255–264 (2002). doi:10.1016/S0001-8686(02)00080-5
Hiemstra, T., Venema, P., Van Riemsdijk, W.H.: Intrinsic proton affinity of reactive surface groups of metal (hydr)oxides: the bond valence principle. J. Colloid Interface Sci. 184, 680–692 (1996). doi:10.1006/jcis.1996.0666
Vogelsberger, W.: Thermodynamic and kinetic considerations of the formation and the dissolution of nanoparticles of substances having low solubility. J. Phys. Chem. B 107, 9669–9676 (2003). doi:10.1021/jp030347z
Lencka, M.M., Riman, R.E.: Thermodynamic modeling of hydrothermal synthesis of ceramic powders. Chem. Mater. 5, 61–70 (1993). doi:10.1021/cm00025a014
Wolfram, S.: Das Mathematica-Buch. Addison-Wesley/Longman, Bonn (1997)
Grenthe, I., Wanner, H., Östhols, E.: TDB-2, Guidelines for the Extrapolation to Zero Ionic Strength. OECD Nuclear Energy Agency (2000)
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Schmidt, J., Vogelsberger, W. Aqueous Long-Term Solubility of Titania Nanoparticles and Titanium(IV) Hydrolysis in a Sodium Chloride System Studied by Adsorptive Stripping Voltammetry. J Solution Chem 38, 1267–1282 (2009). https://doi.org/10.1007/s10953-009-9445-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-009-9445-9