Abstract
The current work involves the use of flash auto combustion procedure to synthesize nano-ferrites Mn0.6Zn0.4LaxFe2-xO4, (x = 0.00, 0.02, 0.04, 0.06, 0.08, and 0.10) annealing at 500 °C for 4 h. X-ray Diffraction (XRD), Fourier transition infrared spectroscopy (FTIR), and high-resolution transmission electron microscopy (HRTEM) were used to characterize the structural properties of produced samples. Scanning electron microscopy (SEM) was utilized to examine the surface morphology of the samples at various Lanthanum concentrations. From XRD, the spinel cubic structure for all samples with few traces of secondary phase at high La concentrations is assured. The crystallite size is estimated to be in the nanoscale range of 13.16–18.13 nm using the Debye–Scherrer formula. The appearance of characteristic vibrational bands near 460 cm−1 and 563 cm−1, which correspond to the octahedral and tetrahedral sites, respectively, confirms the formation of the spinel structure. SEM micrographs show that the grains are nearly irregular in shape, and the accumulation of La+3 ions at the grain boundaries exerts tensile strength and pressure on the grain itself, reducing the grain size. The particle size estimated by TEM coincides well with the crystallite size determined by XRD. The thermogravimetric analysis, (TGA), was used to investigate the thermal properties of the nanoferrites from room temperature to 1000 °C. In comparison to the other samples, the sample with x = 0.04 has greater thermal stability and the TEM measurement indicates that this sample has the smallest particle size. Therefore, we can assert that the thermal stability improves as the particle size decreases. The magnetic permeability was measured in the temperature range 303–773 K at a fixed frequency of 10 kHz and at various La contents. The sample with x = 0.04 has a minimal permeability value, showing that the separation brought on by La ions has diminished the super exchange contact between magnetic ions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nano-spinel ferrite materials have become attractive and marvelous subject in versatile expanding vistas that are useful for investigators for inspection of medicine, manufacturing and academic demands imputing to their stunning peerless physical, ferrimagnetic, chemical, and thermal merits that differ from their bulk ferrite materials. The properties of such nanospinels are determined by their manufacturing process, their chemical arrangement, and the internal arrangement of nanocrystals and nano-sized crystallites. Spinels in nano-configuration have proven to be one of the most useful types of materials for use, owing to a significant change in their physical properties caused by substitution with various metallic cations [1, 2]. The chemical composition, type of precursor, preparation method, annealing, presence of doping elements, impurities, or secondary phases all have a significant impact on the structural and magnetic properties of ferrites [3]. Given that the final properties of nanoparticles are determined by material morphology, which is determined by nanoparticle size, dispersion, and distribution through the matrix, material preparation techniques have advanced rapidly in recent years to support a wide range of applications [4]. Soft spinel ferrite materials have been synthesized using a variety of methods including chemical co-precipitation [5], hydrothermal method [6], mechano-chemical method [7], microemulsion method [5], rheological phase reaction method [8], sol–gel method [9], flash auto combustion method [10], and thermal decomposition of some precursors [11]. The co-precipitation method is widely used because it produces nanoparticles with uniform size distribution and allows for simple control of synthesis parameters and nucleation growth [12]. Multi-component spinel oxides of high purity and homogeneous composition can be obtained using the sol–gel method [12]. Because of their high permeability and minimal magnetic losses at high frequencies, Mn-Zn ferrites are widely used in electronic applications. The properties of ferrites depend generally on their microstructure, so the nano-technique is presented to control their ultra-microstructure, rather than the traditional ceramic way [13]. The electrical and magnetic properties of ferrite may be altered by rare-earth elements “RE” with normal relaxation characteristics. A variety of investigations have been carried out to determine the impact of RE on the spinel structure’s electromagnetic wave retention characteristics. RE3+ ions partial substitution of the 4f elements series for Fe3+ in Mn-Zn ferrite is promising to manage structural, electrical, and magnetic properties. Among the lanthanides, lanthanum has been broadly inspected for its interesting properties. Ferrites have very vital applications concurring to their electrical and magnetic properties. The proportion impact and synthetization condition alert are permitted to progress some ferrites with diverse composition to be utilize in wide frequency extend, from microwaves to electromagnetic radiation [13]. Md. Mojahid Roni et al. [14] have been studied the Synthesis of La-doped Mn0⋅6Zn0.4LaxFe2-xO4 by the conventional solid-state reaction and the study of its structural, electrical, and magnetic properties for high-frequency applications. The cubic spinel structure of Mn0.6Zn0.4LaxFe2-xO4 (x = 0.00–0.10) is confirmed by the XRD pattern. The value of crystallite size “D” determined using the Scherrer relation is 32.94 nm for the unmodified Mn-Zn ferrite series. It shows a decrement trend for D up to x = 0.06 and an increase for x = 0.08–0.10. Prashant Thakur et al. [15] have been used the coprecipitation method to synthesize a series of Mn0.5Zn0.5LaxFe2−xO4, (x = 0, 0.025, 0.050, 0.075, 0.1). The crystallite size decreases to a minimum for x = 0.050 and increases for x > 0.050. The lattice constant and interplanar spacing both have a minimum at x = 0.050. The formation of a spinel ferrite structure is confirmed by FTIR, and there is a shift in the position absorption bands with La doping.
The introduction of La ions affects the structural properties of the studied ferrite up to certain limit and using a new preparation method, consider the novel study of ferrite prepared. Our objective is the successful preparation of the ferrite system Mn0.6Zn0.4LaxFe2-xO4 by flash auto combustion method which gives the facility of nanomagnetic particle to be exposed to high-temperature combustion during the preparation, so the particle size is relatively high compared with co-precipitation method, whereas the magnetic properties are higher than the other methods of preparation. Also to determine the solubility limit of La ions within the ferrite lattice and conduct systematic research on the structure, molecular, morphological, and thermal properties of Mn0.6Zn0.4LaxFe2-xO4 samples.
2 Experimental Details
A series of Mn0.6Zn0.4LaxFe2-xO4 (x = 0.00 to 0.10, with step 0.02) was synthesized to utilize the flash auto combustion technique [16]. The samples were inspected by diffraction employing a Philips model (PW-1729) diffractometer (Cu-Kα radiation source with λ = 1.540598 Å) with 2Ө within the run from 24 to 80°. Infrared spectra for the prepared samples were allotted at room temperature employing a PERKIN-ELMER-1430 where the recording infrared spectra were within the range 200–4000 cm−1 (at Tanta University, Central lab.). The microstructure of the test samples was analyzed employing a scanning electron microscope (SEM (JOEL, Model: JSM-5600, Japan.)). We use the TEM model (Transmission Electron Microscope JEOL- JEM- 2100) to determine the particle sizes for all samples from the captured images. Thermogravimetric analysis (TGA and its derivative) of those nano-ferrites uses instrument model (SDT Q600 V20.9 Build 20). Using RLC Bridge of type BM591 from Tanta University, the prepared samples’ permeability was measured at temperatures ranging from room temperature to > 700 K.
3 Results and Discussion
3.1 X-ray Structural Studies
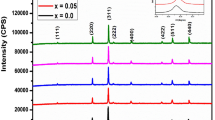
Figure 1 displays the XRD of Mn0.6Zn0.4LaxFe2-xO4 (x = 0.00, 0.02, 0.04, 0.06, 0.08, and 0.10) system prepared by the flash auto combustion method and was annealed at 500 ℃ for 4 h. The pattern confirms the spinel cubic structure in all samples, with only minor traces of secondary phase at high La content. Further, the observed diffraction peaks could be assigned to the reflection plane of (220), (311), (400), (422), (511), and (440) that matched well with JCPDS file no. 74–2401 for the spinel cubic phase [17]. Meanwhile, the peak corresponding to 2θ = 32° is attributed to the secondary phase for LaFeO3 at the grain boundaries accumulated [18] and the intensity of this peak is increased by increasing La3+ content. The presence of a secondary phase of LaFeO3 compound (JCPDS 75–0541) in Mn-Zn-La ferrite nanoparticles implies that the solubility of La3+ ions in the spinel lattice is limited. Similar results have also been reported indicating the smaller solubility of La3+ in ferrites [14, 19,20,21]. The lattice parameter was estimated utilizing the equation [22]. The variation of the lattice parameter as a function of the content of La is represented in Fig. 2. It is found that the lattice parameter increases by increasing La+3 content up to x = 0.04 and then at x = 0.06 decreases and after that increases again may be on account of the large ionic radius of La+3 (1.06 Å) than the iron radius (0.64 Å) so, it is not easy for La3+ ions to replace Fe3+ ions at B sites. In addition, bond energy of La3+- O2− is greater than Fe3+–O2− so, more energy is needed for La3+ to enter the lattice [17]. As a result, a few of La3+ ions accumulate on the grain boundaries which exert pressure on the grains and thus, hinder their growth. In addition, due to larger size of La3+ ions, internal stress is also produced which further obstructs the grain growth and leads to smaller grain size of La substituted Mn-Zn ferrite which causes asymmetry within the structure [23, 24]. The La ions inter the ferrite lattice up to x = 0.04 (solubility limit). The large La ionic radius gives rise to the lattice parameter. At x = 0.06, the reason for the decrease of lattice parameter is related to the grain size value. Above x = 0.06, the accumulation of La on the cell boundary exerts stress on the cell leading to the decrease of the lattice parameter. Similar results have also been reported [17]. In order to calculate \({r}_{A}\) and \({r}_{B}\) the following cation distribution is proposed and given in Table 1:
where t is the traces of Mn present at octahedral site. There is a cation preference for a few ions as an example Zn2+ conquer tetrahedral (A) sites, Mn2+ ions and Fe3+ ions conquer tetrahedral (A) sites and octahedral (B) sites, La3+ exclusively conquer B sites on account of their larger ionic radius. The ionic radius of tetrahedral and octahedral site (rA and rB) respectively was calculated utilizing the subsequent equations [15].
where CA and CB point out the ionic concentration at A and B sites respectively while \({r}_{{\mathrm{Mn}}^{2+}}\), \({r}_{{\mathrm{Zn}}^{2+}}\), \({r}_{{\mathrm{Fe}}^{3+}}\), and \({r}_{{\mathrm{La}}^{3+}}\) are ionic radii of Mn2+, Zn2+, Fe3+, and La3+ ions respectively. The worth of rA and rB is tabulated in Table 2. Theoretical lattice parameter (ath) was estimated using the equation [25]. La3+ ions introduce at interstitial position and cause the unit cell to dilate. At low La3+ content, the worth of aexp near the worth of ath as appeared in Fig. 2 which means that the La3+ are soluble within the lattice, where at high La content there are some dispersions appear because of the non-coincidence among aexp and ath is owing to the existence of LaFeO3 phase. Oxygen positional parameter (U) and inversion parameter (δ) are depicted since the deviation of U from Uideal (without any doping ions) (0.375) is given by next equations [26] and given in Table 2.
The deviation of U from Uideal could be a measure of effect of trigonal distortion at sub-lattices on account of oxygen ion motion. The size of octahedral site is greater than the tetrahedral site, and there is a connection among the octahedral site and oxygen ions. It is not easy for the metal ions to overcome the tetrahedral site owing to small size, so a few adjustments occur by the movement of oxygen ions. Some shrinkage and expansion happens among octahedral and tetrahedral sites on account of this motion that is known as oxygen positional parameter. The theoretical density was estimated utilizing the formula [3]:
where M is the molecular weight, NA is the Avogadro number “(6.022 × 1023 mol−1)” and a is the lattice constant. The theoretical density increase with increasing La content and is higher than bulk density as seen in Table 2 because of the existence of pores that may be formed during the synthetization process. Calculated values of the theoretical density of Mn0.6Zn0.4LaxFe2-xO4 (x = 0.00, 0.02, 0.04, 0.06, 0.08, and 0.10) are 5.2108, 5.2187, 5.2746, 5.2952, 5.3047, and 5.2967 g/cm3 respectively as given in Table 2, which depicts consistency with the values of other reporters [14]. The following equation utilized to calculate the porosity (P) [27]:
where ρ and ρx are the bulk and theoretical (X-ray) densities, respectively. The porosity increases with increasing La content owing to the spaces and pores within the sample synthetization. The values of porosity are in agreement in its trend with the previous study [14]. The crystallite size of synthesized samples was appraised from the broadening of the X-ray peak (311) utilizing Scherer’s equation [3] as illustrated in Table 2. Up to x = 0.04 the crystallite size decreases owing to the large size mismatch among La3+ and Fe3+ that actuates the crystalline anisotropy amid the substitution of ions that makes the inner strain, Subsequently, the crystallite size decreases to diminish the volume strain. Over x = 0.04 La content the crystallite size increase with increasing La content. The appearance of the new phase LaFeO3 could also be the predominant factor for the increasing of crystallite size on account of the distinction in thermal expansion coefficients for these phases. The large amount of La may exert drag forces on boundary motion and grain growth, resulting in a reduction of crystalline size up to x = 0.04. The removal of defects, the alleviation of internal stresses, and the activity of the crystallization process may be related to the rise in crystalline size values when more La content (x > 0.04) is added [14]. The same attitude for crystallite size is reported in [14].
3.2 FTIR Analysis
FTIR spectra for ferrite system within the domain from 200 to 4000 cm−1 are displayed in Fig. 3 that is characterized by the existence of two strong absorption bands, ν1 “for tetrahedral site” and ν2 “for octahedral site” about 563 and 460 cm−1 respectively as tabulated in Table 3. The formation of the spinel structure of the ferrite system was assured by FTIR analysis. These absorption bands affirm the spinel structure and are on account of the stretching vibration of Fe+3–O−2 ion complex [28, 29]. The difference of (ν1) and (ν2) absorption bands intensity is because of the changes within the bond length of Fe3+–O−2 at “A sites” and “B sites” [30, 31]. It is monitored that absorption band (ν1) intensity increment with La3+ ion content, while band (ν2) decreases. The rise and reduction of Fe3+ population result in a mutation within the mode number of the vibration at both tetrahedral and octahedral sites, owing to the decrease and increase of the absorption band intensity. The variation in the intensity of (v1) is caused by the nature of the lanthanum ion, which prefers to conquer the B site and obliged Fe+3 ion into A site with increasing La3+ concentration, which increases the radius of the B site and the number of modes of vibration, resulting in an increase in the intensity of the A site, as tabulated in Table 3. Our results are assured by the bond length gained from XRD data that state that the bond length at tetrahedral site increases by increasing La content as tabulated in Table 3. The bond length estimated from XRD affects the vibration strength used to the vibration frequency. The relation between the frequency and bond length is inverse relation. Additionally, it is known that the variation in the position of FTIR spectra peaks is mainly vassal on the amount of substituent, procedure of synthetization, grain size, and also the density of particles [32]. The bands show up around 3400 cm−1 and 1600 cm−1 can be credited to the stretching vibration mode of H–O–H and the bending vibration of water molecule. The weak band appeared at 1443 cm−1 is responsible for the bending vibration of H2O molecules [33]. Utilizing the formula [34], the force constant for Fe+3–O−2 was calculated. From Fig. 4, Ftetra increase with an increment of La content that can be owing to an increment of ν1 that shift to higher frequency within the increment of La concentration. Focta is decreasing that could be the reason of the decrease of ν2 which shift to a lower frequency by increasing La content.
3.3 SEM analysis
The surface morphology of lanthanum-doped manganese zinc nano ferrites was resolved by SEM, as displayed in Fig. 5 which illustrates that the grains are made up of nearly patchy shape grains. The high calcination temperature and magnetic interactions can contribute to grain agglomeration together to produce larger grains [18], and as La ion concentration increases, the agglomeration decreases. The magnetic interaction between the samples’ constituent particles is the cause of the agglomeration [16]. La acts as an inhibitor for dipole exchange interaction which leads to the decrease of magnetic interaction and decrease the agglomeration. Particles at the nanoscale have a larger surface-to-volume ratio, which results in high interfacial surface tension, which is another reason for the agglomeration of particles. La+3 ions accumulation at grain boundaries exerts a tensile strength and pressure on the grain itself that lowers grain size. The average grain size is estimated using the intercept method followed by the equation [33] and it is found to decrease with increasing La content in the range of 32.48 to 56.75 nm as given in Table 2 which is higher than the crystallite size estimated from XRD because the grain size set from SEM is made of many crystallites whereas the X-ray line broadening analysis gives only a single crystallite size [16].
3.4 HRTEM Analysis
The HRTEM of nano ferrite samples Mn0.6Zn0.4LaxFe2-xO4, (x = 0.00, 0.02, 0.04, 0.06, 0.08, 0.10) are represented in Fig. 6. The image displays that the particles majority are spherical and agglomerated. The crystallite size (D) set from the XRD study agrees well with that determined by TEM as tabulated in Table 2. The SEM micrograph describes the grain at the surface of the samples and each grain consists of many particles oriented in a specific direction. The particle size estimated from TEM is higher than the crystallite size deduced from Scherer’s equation due to the agglomeration occurred for magnetic nanoparticles. So grain size > particle size > crystallite size as given in Table 2. The monitored agglomeration could be caused by magnetic interactions among nanoparticles [17]. Otherwise, the La3+ non-magnetic quality could expound wherefore agglomeration lowers with rising La substitution. After volume expansion and supersaturation reduction, nanoparticles may grow at different rates, resulting in differences in size and morphology [35]. The observed crystallographic values of 0.21 nm and 0.16 nm correspond to the lattice spaces of the (400) and (511) plans of the ferrite system, respectively as shown in Fig. 6B. The noticed crystallographic d qualities concur with those acquired from XRD examination, so the TEM and XRD affirm the arrangement of spinel phase of ferrite [36, 37]. The selected area electron diffraction (SAED) pattern shows concentric rings which confirm the crystalline structure of the given ferrite as shown in Fig. 6C [37].
3.5 Thermogravimetric Analysis (TGA)
Figure 7 displays the thermodynamic curves for ferrite samples in the temperature from room temperature to 1000 °C. TGA plots manifestation weight loss within a specific temperature “T” as tabulated in Table 4; this temperature is considered as the initial decomposition of the organic component and has a distinct value for various La concentration and shift to riser temperature reaching a maximum at x = 0.04 La content and then decreasing, so the sample x = 0.04 has the greatest thermal stability compared with the other. The increase of La content above x = 0.04 leads to the appearance of LaFeO3 phase which has a thermal expansion coefficient (TEC) equal to \(9.7\times {10}^{-6} {K}^{-1}\) [38] which is lower than the value of TEC for spinel ferrite equal to \(14.4\times {10}^{-6} {K}^{-1}\) [39]. During the preparation, the distinction of TEC for both phases causes the growth of grain size by different values. The doping of La ions in the ferrite lattice causes the increase of thermal stability up to x = 0.4. Above this value, the La ions are accumulated on the cell boundary leading to the formation of the secondary phase LaFeO3 which will have low thermal stability causing the decrease of the specific temperature as given in Table 4. The increase of thermal stability means the initial decomposition of ferrite becomes at a high temperature. As the size of the nanoparticle decreases, the cohesive energy and melting point gradually decrease, so the structural and thermal stability deteriorate [40]. As illustrated in Fig. 7, the TGA curve has three stages owing to weight loss: the primary stage from room temperature till 400 °C, where weight losses were imputed to H2O volatilization within the nano spinels over the rise of heating [41]. That was assured by small endothermic conversions among room temperature up to ~ 400 °C by derivative weight loss plots as illuminated in Fig. 7 but the second from 400 up to 800 ℃ is on account of the abstraction of volatile product and complete degradation of either organic phase existing in the sample pending preparation by flash method [42]. Owing to the complete degradation of the organic material in the prepared samples, a novel stage viewed over 800 °C.
3.6 Magnetic Permeability
As a function of temperature, the magnetic permeability was measured in the temperature range 303–773 K at a fixed frequency of 10 kHz and at various La contents, as shown in Fig. 8. When the material changes from a ferrimagnetic to a paramagnetic state, which occurs at a temperature close to the Curie temperature Tc, the initial permeability abruptly decreases [43, 44]. As the La content rises to a particular worth, the Tc starts to rise instead of decrease as shown in Fig. 9. The idea that La ions take interstitial position among ferric ions after substituting for Fe ions up to a particular solubility limit at x = 0.04 is how these results are explained. Also, the sample with x = 0.04 has a minimal permeability value, showing that the separation brought on by La ions has diminished the super exchange contact between magnetic ions. Following that, the super exchange interaction is not yet affected by the altered interstitial position of La ions, which enhances the magnetic exchange interaction, leading to an increase in permeability at samples x = 0.06, 0.08, 0.10 [45]. At the octahedral site, the substitution of La ions for Fe+3 ions results in a decrease in the strength of the exchange interaction between the A and B sites, which in turn results in a decrease in permeability and Tc. Above these limits, the number of magnet ions at the octahedral site is unaffected by the presence of La ions, resulting in an increase in the magnetic exchange interaction and subsequent rises in permeability and Curie temperature. Saturation magnetization (Ms), crystallite size (D), and the magnetic anisotropy constant (K) all have an impact on permeability. The relation [33] can be used to calculate the initial permeability:
Crystallite size clearly affects the permeability because they behave similarly. Up to x = 0.04 the crystallite size decreases and over x = 0.04 La content the crystallite size increase with increasing La content.
4 Conclusions
Nano-ferrite Mn0.6Zn0.4LaxFe2-xO4, (x = 0.00 to x = 0.10 with step 0.02) were synthesized utilizing flash auto combustion method which is considered a new successful method of preparation which give the facility of nanomagnetic particle to be exposed to high-temperature combustion during the preparation. The effect of La3+ substitution for Fe3+ in Mn-Zn ferrite was investigated. This paper is applying XRD, FTIR, SEM, TEM, and TGA analysis and magnetic permeability respectively. XRD patterns assured the spinel cubic structure in all samples, with only minor traces of secondary phase (LaFeO3) at high La content. Numerous microstructural parameters were evaluated from XRD such as crystallite size, lattice constant, X-ray density, bulk density, and porosity. The large amount of La may exert drag forces on boundary motion and grain growth, resulting in a reduction of crystalline size up to x = 0.04. The formation of the spinel structure of the ferrite system was assured by FTIR analysis. La acts as an inhibitor for dipole exchange interaction which leads to the decrease of magnetic interaction and decrease the agglomeration. In the range of 32.48 to 56.75 nm, the average grain size decreases with increasing La content. The TEM and XRD confirm the spinel phase of ferrite’s arrangement since the observed crystallographic values of 0.21 nm and 0.16 nm correspond to the lattice spaces of the (400) and (511) planes, respectively. The doping of La ions in ferrite lattice causes the increase of thermal stability up to x = 0.4 and above this value, the La ions are accumulated on the cell boundary leading to the formation of the secondary phase LaFeO3. Up to x = 0.04 La content, the permeability decrease and over x = 0.04, it increases with increasing La content. As the La content rises to a particular value, Tc starts to rise instead of decrease. In general within La ions, the solubility limit affects the structural and physical properties of ferrite material in a wide range.
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
References
Lenin, N., Kanna, R.R., Sakthipandi, K., Kumar, A.S.: Mater. Chem. and Phys. 212, 385–393 (2018)
Amer, M.A., Matsuda, A., Kawamura, G., El-Shater, R., Meaz, T., Fakhry, F.: J. Magn. Magn. Mater. 439, 373–383 (2017)
Dippong, T., Levei, E.A., Deac, I.G., Goga, F., Cadar, O.: J. Anal. Appl. Pyrolysis 144, 104713 (2019)
Dippong, T., Levei, E.A., Tanaselia, C., Gabor, M., Tudoran, L.B., Borodi, G., Nasui, M.: J. Magn. Magn. Mater. 410, 47–54 (2016)
Abdullah Dar, M., Shah, J., Siddiqui, W.A., Kotnala, R.: K.: Appl. Nanosci. 4, 675–682 (2014)
Köseoğlu, Y., Bay, M., Tan, M., Baykal, A., Sözeri, H., Topkaya, R., Akdoğan, N.: J. Nanoparticle Res. 13, 2235–2244 (2011)
Yang, H., Zhang, X., Ao, W., Qiu, G.: Mater. Res. Bull. 39, 833–837 (2004)
Jing, J., Liangchao, L., Feng, X.: J. Rare Earths 25, 79–83 (2007)
Hussain, A., Abbas, T., Niazi, S.B.: Ceram. Int. 39, 1221–1225 (2013)
Hemeda, O.M., Tawfik, A., Mostafa, M., Zaki, M., Abd El Ati, M.I.: IOP Conf. Series J. Phys. Conf. Series 1253, 012026 (2019)
Stefanescu, M., Stoia, M., Stefanescu, O., Dippong, T., Barvinschi, P.: Acta Chim. Slov. 56, 379–385 (2009)
Dippong, T., Cadar, O., Levei, E.A., Deac, I.G., Goga, F., Borodi, G., Barbu-Tudoran, L.: Ceram. Int. 45, 7458–7467 (2019)
D’souza, A., Deepak Kumar, M., Chatim, M., Naik, V., Naik, P.P., Tangsali, R.: B. Adv. Sci. Lett. 22, 773–779 (2016)
Mojahid Roni, Md., Hoque, K., Paul, T.C., Khan, M.N.I., Emran Hossain, Md.: Results Mater. 11, 100215 (2021)
Thakur, P., Sharma, R., Kumar, M., Katya, S.C., Negi, N.S., Thakur, N., Sharma, V., Sharma, P.: Mater. Res. Express 3, 075001 (2016)
Mostafa, M., Salem, B.I.: Mater. Sci. Eng. B 286, 116043 (2022)
Phor, L., Kumar, V.: Ceram. Int. 45, 22972–22980 (2019)
Dasan, Y.K., Guan, B.H., Zahari, M.H., Chuan, L.K.: PLoS ONE 12, 0170075 (2017)
Roy, P.K., Bera, J.: Mater. Res. Bull. 42, 77–83 (2007)
Ur Rahman, A., Rafiq, M.A., Karim, S., Maaz, K., Siddique, M., Hasan, M.M.: Physica B Condens. Matter 406, 4393–4399 (2011)
Rezlescu, E., Rezlescu, N., Popa, P.D.: J. Magn. Magn. Mater. 290–291, 1001–1004 (2005)
Sridharl, R., Ravinder, D., Kumar. K.V.: Adv. Mater. Phys. Chem. 2, 192–199 (2012)
Sattar, A.A., Samy, A.M., El-Ezza, R.S., Eatah, A.E.: Phys. Status Solidi 193, 86–93 (2002)
Zhao, L., Yang, H., Yu, L., Sun, W., Cui, Y., Yan, Y., Feng, S.: Phys. Status Solidi 201, 3121–3128 (2004)
Babu, K.V., Satyanarayana, G., Sailaja, B., Santosh Kumar, G.V., Jalaiah, K., Ravi, M.: Results Phys. 9, 55–62 (2018)
Al-Juaid, A.A., Gabal, M.A.: J. Mater. Res. Technol. 14, 10–24 (2021)
Ahmad, I., Farid, M.T., Kousar, R., Niazi, S.B.: World Appl. Sci. J. 22, 796–801 (2013)
Khairy, M., Gouda, M.E.: J. Adv. Res. 6, 555–562 (2015)
Thangjam, B., Soibam, I.: Adv. Mater. Proc. 2, 205–208 (2017)
Patil, R., Delekar, S., Mane, D., Hankare, P.: Results Phys. 33, 129 (2013)
Shirsath, S.E., Toksha, B.G., Kadam, R.H., Patange, S.M., Mane, D.R., Jangam, G.S., Ghasemi, A.: J. Phys. Chem. Solids. 71, 1669–1675 (2010)
Ateia, E.E., Abdelatif, G., Ahmed, M., Mahmoud, M.A.A.: J. Inorg. Organomet. Polym. Mater. 26, 81–90 (2016)
Altarawneh, A.M., Arrasheed, E.A., Ajlouni, A.W., Ghazy, R., Hemeda, O.M., Henaish, A.M.A., Mostafa, M.: Ceram. Int. in press (2023)
Hemeda, O.M., Salem, B.I., Mostafa, M.: Eur. Phys. J. Plus 135, 46 (2020)
Dippong, T., Deac, I.G., Cadar, O., Levei, E.A.: J. Nanomater. 11, 2232 (2021)
Mondal, B., Kundu, M., Mandal, S.P., Saha, R., Roy, U.K., Roychowdhury, A., Das, D.: ACS Omega 4(9), 13845–13852 (2019)
Jahan, N., Khan, M.I., Khandaker, J.I.: ACS Omega 6(48), 32852–32862 (2021)
Løken, A., Ricote, S., Wachowski, S.: Crystals 8, 365 (2018)
Liu, C.W., Lin, C.H., Fu, Y.P.: J. Am. Ceram. Soc. 90(10), 3349–3352 (2007)
Wang, G., Xu, Y.-S., Qian, P., Su, Y.-J.: Comput. Mater. Sci. 169, 109090 (2019)
Ghoneim, A.I., Meaz, T.M., Aboelkhir, H.A.: IOP Conf. Series: J. Phys.Conf. Series. 1253, 012020 (2019)
Henaish, A.M.A., Mostafa, M., Salem, B.I., Hemeda, O.M.: Ph. Transit. (2020). https://doi.org/10.1080/01411594.2020.1758322
Hemeda, O.M., Mostafa, N.Y., Abd Elkader, O.H., Hemeda, D.M., Tawfik, A., Mostafa, M.: J. Magn. Magn. Mater. 394, 96 (2015)
Henaish, A.M.A., Hemeda, O.M., El Refaay, D.E., Mohamed, S., Hamad, M.A.: J. Magn. Magn. Mater. 561, 169736 (2022)
Hossain, M.D., Jamil, A.T.M.K., Hasan, M.R., Ali, M.A., Esha, I.N., Hossain, M.S., Hakim, M.A., Khan, M.N.I.: Mater. Res. Express 8, 046102 (2021)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
This article does not contain any studies with human or animal subjects.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mostafa, M., Khalaf, A., El-Maghraby, E.M. et al. The Impact of La Substitution on the Structural, Molecular, Morphological, and Thermal Properties of Mn0.6Zn0.4Fe2O4. J Supercond Nov Magn 36, 1277–1290 (2023). https://doi.org/10.1007/s10948-023-06564-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-023-06564-x