Abstract
Herein, we report the study of half-Heusler ScTiX (X = Si, Ge, Pb, In, As, and Tl) compounds for the first time employing the scheme of density functional theory (DFT). The influence of on-site Coulomb interactions is taken into account, and simulations are conducted in generalized gradient approximation with the added Hubbard U term (GGA + U). All the compounds were observed to have a narrow band gap on the spin-down configuration. Though spinning the majority channel (spin-up), it is found to be metallic. Consequently, all compounds are semi-metallic or half-metallic and 100% of spin polarized at the Fermi level. Various features, comprising structural, magnetic, elastic, and electronic properties, are calculated through full-potential linearized augmented plane wave (FP-LAPW) method, since they are incorporated in the computer simulation package of WIEN2k. Equilibrium lattice constants are observed for all the compounds which exist within the domain of 6.4–6.8 A°. The IRelast package is already integrated in WIEN2K that has been used for the elastic properties. Elastic features reflect the brittle character of all the material. The total magnetic moments for all such materials are greater than 3 μB, i.e., 3 μB of MTot. Therefore, the compounds show a strong ferromagnetic behavior. These are therefore expected to be used as shape base for thin layers within metastable situations for spintronic applications. The resulted elastic properties show that ScTiSi is ductile, while all other five compounds represent brittle nature. Above findings delight the prospect of ScTiX (X = Si, Ge, Pb, In, As and Ti) compounds in developing half-metallic HH compounds for spintronics and memory storage appliances.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The half-metallicity aspect is caused by an exceptional phenomenon of electronic material structure. Half-metal with opposite spin for electrons is acting differently. Half-metallic compounds behave metallic for electrons with a specific spin and insulating or semiconducting for electrons of the opposite spin. Spintronics is a growing field of nanoscale electronics which uses an electron spin, instead of an electrical charge, encoding and storing facts [1,2,3]. These alloys with full spin polarization at Fermi stage are extremely valuable for spintronics uses due to their high-spin polarization [4, 5]. Such variety of material has an ample and amazing interest for scientific researchers has owing to their prospective information technology, for instance, non-volatile magnetic random access memory (MRAM) and magnetic sensors [4, 6]. The first half-Heusler compounds was NiMnSb discovered by de Groot et al. in 1983 [7]. They exist in different classification system, like full- and half-Heusler compounds [8, 9]. It has four FCC sublattices and is commonly crystallized in the L21 and C1b structures correspondingly. Full- and half-Heusler ternary alloys have X2YZ and XYZ common patterns, where X/Y are high-/low-valent transition metals and Z signifies sp. electron atom. Diminution of the symmetry separates the X2 sublattice which tends to result in the XYZ half-Heusler formula. The half-metallic weak ferromagnetic half-Heusler compounds have been observed in a wide variety of materials. Nasir et al. [10] studied and reported different physical features of HH compounds RhCrZ (Z = Si, Ge). The HH compounds XYZ (X,Y = V, Cr, Mn, Fe, Co, and Ni; Z = Al, Ga, In, Si, Ge, Sn, P, As, and Sb) were reported by L. Feng et al. [11] using the ab initio study. Kulwinder Kaur and Ranjan Kumar performed the high-temperature thermoelectric behavior of p-type TaRhSn half-Heusler compound as vide in [12]. Half-Heusler (HH) substances are significant high-temperature thermoelectric (TE) materials and also have endeared recently a great consideration. Chenguang Fu et al. investigated the high band degeneracy bestows to high thermoelectric performance in p-type HH compounds as can be vide in [13]. Giri Joshi et al. studied an N-type HH compound by the construction of nanocomposite as shown in [14]. In this paper, the structural, elastic, electronic, and magnetic features of half-Heusler ScTiX (X = Si, Ge, Pb, In, As, and Tl) alloys are studied under density functional theory (DFT), as incorporated in simulation package of WIEN2K.
2 Simulation Methodology
The FP-LAPW method [15] incorporated in WIEN2K package [16] is used for the simulation of the compounds. The influence of on-site Coulomb interactions is taken into account, and simulations are conducted in GGA with the added Hubbard U term (GGA + U) [17]. Through the spin-polarized density functional theory (SPDFT), electronic structure evaluation and the optimization of geometry are carried out by the FP-LAPW method. To compute densities with together spin-up (↑) and spin-down (↓) states channel, the Kohn-Sham systems of equations are resolved self-consistently [18, 19]. The energy difference of – 6 Ry is taken within the core and valence states. Inside the muffin-tin-spheres, the spherical harmonic functions with cut-off l-max = 10 are used. Moreover, the RKmax is 2.5, 2000 is K-points in order to gain better and confident results and chosen G-max to be 12. Structural parameters are analyzed by adapting the energy versus volume curve employing Murnaghan’s state equation [20]. The package IRelast, modeled by M. Jamal [21], is already integrated in WIEN2K which has been used for elastic constants (ECs) of crystals with various symmetries, such as cubic, which are exploited for the analysis of elastic and mechanical properties depending on second-order energy-derived vs zero strain.
3 Results and Discussion
We present a thorough description of various features of HH compounds ScTiX (X = Si, Ge, Pb, In, Sb, and Tl).
3.1 Structural Properties
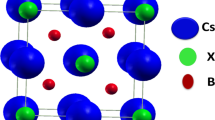
It has been found from the measurement that the material of ScTiX (X = Si, Ge, Pb, In, Sb, and Tl) crystallizes into the cubic structure of type MgAgAs which is compatible to the space group F-43 m (216). In these compounds, Sc settled at (1/2, 1/2, 1/2, 1/2); Ti occupies (1/4, 1/4, 1/4); and X = Si, Ge, Pb, In, Sb, and Tl hold (0, 0, 0) Wyckoff positions. ScTiX prototype atomic structural arrangement is shown in Fig. 1.
For the computation of ground-state structural properties, the total energy vs volume is fitted by Burch-Murnaghan equation of state [20]. From the Murnaghan fit curve, the optimized structural parameters are estimated as shown in Fig. 2. The point with minimum energy relative to volume depicts the state of the system at equilibrium, and volume is known to be optimum volume or ground-state volume and is employed for the computation of ground-state lattice parameter.
The results of structural parameters are given in Table 1.
3.2 Electronic Properties
To calculate the electronic nature of these compounds, the bands configuration in addition to density of states (DoS) of ScTiX (X = Si, Ge, Pb, In, Sb and Tl) for both spin-up and spin-down configuration are simulated. Band gap and DoS for spin-polarized electronic channel are measured by considering the influence of on-site Coulomb interactions, and simulations are conducted in GGA + U.
Electronic DOS structures of all our compounds are presented in Fig. 3 for both spin-up and spin-down case. It can be seen from the figures that Sc_d,Ti_d and X = (Si, Ge, Pb, In, Sb, and Tl)_s,p states contribute and crossover the Fermi level in spin-up configuration dominantly to the electronic DOS and result into a metallic behavior of the all compounds. In spin-down case, Sc_d,Ti_d and X = (Si, Ge, Pb, In, Sb and Tl)_s, p states contribute to the DOS structure but do not crossover the Fermi level which result into a band gap at the Fermi level and hence produce a half-metallic behavior.
Figure 4 manifests band structures in spin-polarized case for ScTiX (X = Si, Ge, Pb, In, Sb and Tl) for the majority spin (spin-up (↑)) and minority spin (spin-down (↓)) electrons in the Brillouin zone. It is pretty clear that in the spin-up majority platform, the metallic behavior of all the compounds is depicting from overlapping bands, and a band gap exists there, showing the semiconductor existence of all the compounds in the minority (spin-down) track, as can be seen from the Fig. 4. In spin-down channel, all the compounds have indirect narrow band gaps of 0.32, 0.79, 0.43, 0.12, 1.62, and 0.31 eV, for ScTiSi, ScTiGe, ScTiPb, ScTiIn, ScTiSb, and ScTiTl, accordingly; somewhere the majority of the valence band appears at the Γ-point, and conduction band minima is at the X-point resulting an indirect band gap from Γ-X. We for that reason deduce that the compounds ScTiX (X = Si, Ge, Pb, In, Sb and Tl) possess half-metallic behavior. The gap leads to a 100% spin polarization at the Fermi level energy EF, which is a consequence that it results in the half-metallic nature of the compounds. The thickness of the energy difference is turned to be sensitive to the lattice parameters. We understand that the lower Coulomb repulsive force values for lightweight elements are accountable for the small band gap.
3.3 Magnetic Properties
In accordance with the Galanakis model [22, 23], the spin magnetic moment of HH compositions is equivalent to the variation between the occupied bands in the up and down spin states. HH ternary 1:1:1 alloys have a C1b structure; its performance such as magnetic, non-magnetic, and semiconducting can be easily evaluated from the Slater-Pauling equation [24], presented as Mt = (18 − Zt) μB for orbitals hybridization in which Mt represents the total magnetic moment and Zt appear for the total number of valence electrons in the unit cell.
Here in this case, the electronic configuration of integral atoms are Sc [3d14s2] and Ti [3d24s2], for (X = Si, Ge, Pb, In, Sb, and Tl) and Si [3s23p2], Ge [4s24p2], Pb [6s26p2], In [5s25p1], and Sb [5s25p3] for Tl [6s26p1]. As, all of our compounds, has valence electrons less than 18, i.e., not 18-valence electron system and thus must have some magnetism. We have shown in Table 2 the total magnetic moment and donations from atomic resolved and interstitial magnetic moments for ScTiX (X = Si, Ge, Pb, In, Sb, and Tl). It can be clearly seen that Ti atom contributes larger to the total magnetic moment of all compounds.
3.4 Elastic Properties
The elastic features of materials are essential for scientific, medical, and engineering uses. Elastic features are therefore important for uses of materials that can be conveniently measured utilizing elastic constants. DFT is quite a flourishing theory and a means for the computation of elastic constants of solids. Elastic constants take part a crucial role in shaping the features of materials and illustrate their reaction when subjected to exterior forces [25,26,27,28]. Elastic constants supply a link to the atomic and the world on a huge scale [29,30,31]. By means of the elastic stability criteria, ECs might be employed to separate elastic from plastic domain [32]. Specific physical properties, such as hardness, modulus of Voigt, modulus of Reuss, modulus of Hill, modulus of Shear, Young’s modulus, Bulk modulus, elastic stiffness ratio, the ratio of Poisson, anisotropy factor A, and the melting temperature are measured through ECs [33].
We have computed the elastic constants (C11, C12, and C44) for the ScTiX (X = Si, Ge, Pb, In, Sb, and Tl) compounds and correlate them with the available literature. Table 3 shows all the elastic parameters for our compounds. The mechanical stability criteria for the cubic compounds is C11 − C12 > 0, C11 + 2C12 > 0, C44 > 0.4, and B > 0 [34]. The mechanical properties, such as Young’s modulus (Y), shear modulus (GH), bulk modulus (B), anisotropy (A), and Poisson’s ratio (ѵ), are determined from such elastic constants for all material. The shear modulus (G) indicates the toughness of the system. All the computed data of the shear moduli for all the studied HH are illustrated in Table 3. It is visible from the table that ScTiIn has the highest shear modulus value of 159.319 GPa of all of these compounds, which illustrate that this compound among all of these is the hardest one. From the table, it can be illustrated that the lowest value of shear modulus is − 104.36 Gpa for ScTiTl, which represents that a very small opposition to the deformation existed for this compound. Young’s moduli E displays the material’s stiffness [35]. Table 3 shows the computed value for all the investigated half-Heusler ScTiX compounds. It can be seen that ScTiIn has 183.841 GPa value of E; this indicates the high stiffness of the material compare with the others. The stiffness of these materials is manifested from the higher values of Young’s modulus relative to the shear modulus. The ductile aspect of the material is quite significant from an engineering perspective and can be calculated by the ratio of B/G. The criteria for ductile material are as follows: B/G is larger than 1.75, and that for brittle material, B/G is lower than 1.75 [36]. The calculated B/G ratio for all ScTiX (X = Si, Ge, Pb, In, Sb, and Tl) is presented in Table 3.
Table 3 expresses that for ScTiSi this ratio is 4.252, i. e., greater than the critical value, and hence the compounds are ductile in nature, while for all other five compounds, the B/G ratio is less than the criteria 1.75, so the other five investigated compounds are founded to be brittle in nature. (C// = C12 − C44) which is a Cauchy’s pressure parameter is employed to characterize the ductility/brittleness nature of the material. The Cauchy’s pressure positive value represents the ductility nature, and the negative value shows the brittleness character of the material. The ratio of Poisson ʋ seems to be a very significant parameter which can be employed to explain the different mechanical properties, i.e., the incompressibility of the material. The standard value of the Poisson ratio is within 0 and 0.5. Incompressibility improves when you go from 0 to 0.5 [37]. The estimated values of the Poisson ratio for all the compounds are shown in Table 3. It is noticeable from the table that ratios of Poisson for materials ScTiSi, ScTiGe, ScTiIn, and ScTiSb are 0.390, 0.228, 0.410, and 0.243, respectively, appertain in the upper range, which is why these compounds are low compressible in response to deformation, while ScTiPb and ScTiTl have 1.807 and 1.750 which are out of the above limit and thus are highly compressible in opposition to deformation. In addition, this rate is often employed to evaluate the existence of the interatomic forces. Anisotropy factor (A) is a parameter that indicates whether or not the material structural properties remain the same in all directions. For the medium to be isotropic, A = 1, and if A is not equal to 1, the medium is anisotropic. The estimated anisotropic index for each of these compounds is shown in Table 3. Computed values for all compounds are listed and vary from unity, which implies that their characteristics differ in different orders; thus, all are anisotropic compounds. The B/C44 which is bulk modulus ratio C44 might work to measure the plasticity of a material [38]. Table 3 displays the values of B/C44 for such compounds. This is noticeable in Table 3 that the ratio B/C44 seems to be the greatest for ScTiSi (1.414 GPa) and the lowest for ScTiTl. The higher value of B/C44 for ScTiSi suggests a high extent of plasticity in contrast to others.
4 Conclusion
We examined ScTiX (X = Si, Ge, Pb, In, Sb and Tl) half-Heusler compounds employing PBE-GGA within WIEN2K simulation package through context of DFT for structural, elastic, electronic, and magnetic properties. From calculated results of the equilibrium lattice constants, the elastic constants and the bulk module B, the shear module G and the Young module E verify such that all compounds exhibit stable structure but mechanically unstable. From the elastic properties, we conclude that ScTiSi is ductile, while all other five investigated compounds are brittle in nature. Electronic band gap and DOS studies indicate that the compounds are semi-metallic with a small indirect band gaps from Γ-X in the spin-down platform. We have found that the magnetic moment of spin is roughly greater than 3, i.e., 3 μB of MTot. It is also shown that the significant participation to magnetic moments results from Ti atom. Since half-metallic HH alloys have special, magneto-optical properties, current research study may possess uses in spintronics. HH alloys are important for thermoelectric, solar and applications diluted magnetic semiconductor. The above finding can be deemed as comprehensive insight in its applications in the field of high-performance electronic and magnetic devices.
References
Shaughnessy, M., Fong, C.Y., Snow, R., Yang, L.H., Chen, X.S., Jiang, Z.M.: Structural and magnetic properties of single dopants of Mn and Fe for Si-based spintronic materials. Phys. Rev. B. 82, 035202 (2010)
Damewood, L., Fong, C.Y.: Local field effects in half-metals: a GW study of zincblende CrAs, MnAs, and MnC. Phys. Rev. B. 83, 113102 (2011)
Shaughnessy, M., Fong, C.Y., Snow, R., Liu, K., Pask, J.E., Yang, L.H.: Origin of large moments in Mn x Si 1 − x at small x. Appl. Phy. Lett. 95, 022515 (2009)
I. Zuti’c, J. Fabian, S. D. Sarma: Spintronics: fundamentals and applications. Rev. Mod. Phys. 76, 323 (2004)
Boeck, J.D., Roy, W.V., Motsnyi, V., Liu, Z., Dessein, K., Borghs, G.: Hybrid epitaxial structures for spintronics. Thin Solid Films. 412, 3–13 (2002)
Wolf, S.A., Awschalom, D.D., Buhrman, R.A., Daughton, J.M., von Molnar, S., Roukes, M.L., Chtchelkanova, A.Y., Treger, D.M.: Spintronics: a spin-based electronics vision for the future. Science. 294, 1488–1495 (2001)
De Groot, R.A., Mueller, F.M., Engen, P.G.V., Buschow, K.H.J.: New class of materials: half-metallic ferromagnets. Phys. Rev.Lett. 50, 2024 (1983)
Hülsen, B., Scheffler, M., Kratzer, P.: Thermodynamics of the Heusler alloy Co2−xMn1+xSi: a combined density functional theory and cluster expansion study. Phys. Rev. B. 79, 094407 (2009)
Lee, S.C., Lee, T.D., Blaha, P., Schwarz, K.: Magnetic and half-metallic properties of the full-Heusler alloys co 2 Ti X ( X = Al , Ga ; Si , Ge , Sn ; Sb ). J. Appl. Phys. 97, 10C307 (2005)
Mehmood, N., Ahmad, R., Murtaza, G.: Ab initio investigations of structural, elastic, mechanical, electronic, magnetic, and optical properties of half-Heusler compounds RhCrZ (Z = Si, Ge). J. Supercond. Nov. Mag. 30, 2481–2488 (2017)
Feng, L., Liu, E.K., Zhang, W.X., Wang, W.H., Wu, G.H.: First-principles investigation of half-metallic ferromagnetism of half-Heusler compounds XYZ. J. Magn. Magn. Mater. 351, 92–97 (2014)
Kaur, K., Kumar, R.: High temperature thermoelectric performance of p-type TaRhSn half Heusler compound: a computational assessment. Ceram. Int. 43, 15160–15166 (2017)
Fu, C., Zhu, T., Pei, Y., Xie, H., Wang, H., Jeffrey Snyder, G., Liu, Y., Liu, Y., Zhao, X.: High band degeneracy contributes to high thermoelectric performance in p-type half-Heusler compounds. Adv. Energy Mater. 4, 1400600 (2014)
Joshi, G., Yan, X., Wang, H., Liu, W., Chen, G., Ren, Z.: Enhancement in thermoelectric figure-of-merit of an N-type half-Heusler compound by the nanocomposite approach. Adv. Energy Mater. 1, 643–647 (2011)
Singh, D.J., Nordstr¨Om, L.: Planewaves, pseudopotentials, and the LAPW method. Springer, New York (2006)
P. Blaha, K. Schwarz, G.K.H. Madsen, D. Kvasnicka, J. Luitz, R. Laskowski, F. Tran, L. D. Marks, WIEN2K, an augmented plane wave + local orbitals program for calculating crystal properties, Karlheinz Schwarz, Techn.Universit¨at WIEN, Austria, Wien, Austria (2001)
Perdew, J.P., Burke, K., Ernzerhof, M.: Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996)
U. von. Barth, L. Hedin.: A local exchange-correlation potential for the spin polarized case. I. J. Phys. C Solid State Phys. 5, 1629 (1972)
Pant, M.M., Rajagopal, A.K.: Theory of inhomogenous magnetic electron gas. Solid State Commun. 10, 1157–1160 (1972)
Murnaghan, F.D.: The compressibility of media under extreme pressures. Proc. Natl. Acad. Sci. U. S. A. 30, 244–247 (1944)
Jamal, M., Bilal, M., Ahmad, I., Jalali-Asadabadi, S.: IRelast package. J. Alloy. Compd. 735, 569–579 (2018)
I. Galankis, P. H. Dederiches, Half-metallic alloys: fundamentals and applications. Springer, Berlin (2005)
Galanakis, I., Dederichs, P.H., Papanikolaou, N.: Slater-Pauling behavior and origin of the half-metallicity of the full-Heusler alloys. Phys. Rev. B. 66, 174429 (2002)
Galanakis, I., Mavropoulos, P., Dederichs, P.H.: Electronic structure and Slater–Pauling behaviour in half-metallic Heusler alloys calculated from first principles. J. Phys. D. Appl. Phys. 39, 765–775 (2006)
Ravindran, P., Fast, L., Korzhavyi, P.A., Johansson, B., Wills, J., Eriksson, O.: J. Appl.Phys. 84, 4891 (1989)
Yu, R., Zhang, X.F., de Jonghe, L.C., Ritchie, R.O.: Elastic constants and tensile properties of Al2OC by density functional calculations. Phys. Rev. B. 75, 104114 (2007)
Łepkowski, S.P., Gorczyca, I.: Ab initio study of elastic constants in InxGa1−xN and InxAl1−xN wurtzite alloys. Phys. Rev. B. 83, 203201 (2011)
Xie, M.Y., Tasnadi, F., Abrikosov, I.A., Hultman, L., Darakchieva, V.: Elastic constants, composition, and piezolectric polarization in InxAl1−xN: from ab initio calculations to experimental implications for the applicability of Vegard’s rule. Phys. Rev. B. 86, 155310 (2012)
Callaway, J.: Quantum Theory of the Solid State, second edn. Academic Press, New York (1991)
Mase, G.T.: G.E. Theory, Applications, and Numerics, second ed., CRC Press LLC, Mase, Elasticity (1999)
P. Bruesch, Phonons: theory and experiments I : lattice dynamics and models of interatomic forces, Springer-Verlag, 1982
M.H. Sadd, Elasticity: theory, applications, and numerics, Elsevier Academic Press, 2005
Yao, H., Ouyang, L., Chingw, W.: Ab initio calculation of elastic constants of ceramic crystals. J. Am. Ceram. Soc. 90, 3194 (2007)
G. Grimvall, Thermophysical properties of materials, Elsevier, Amsterdam 1999, enlarged and revised edition
C. Jenkins, S. Khanna “Mechanics of materials: a modern integration of mechanics and materials in structural design,” 2005
Wachter, P., Filzmoser, M., Rebizant, J.: Electronic and elastic properties of the light actinide tellurides. Phys. B Condens. Matter. 293, 199–223 (2001)
Mott, P.H., Dorgan, J.R., Roland, C.M.: The bulk modulus and Poisson’s ratio of “incompressible” materials. J. Sound Vib. 312, 572–575 (2008)
Vitos, L., Korzhavyi, P.A., Johansson, B.: Stainless steel optimization from quantum mechanical calculations. Nat. Mater. 2, 25–28 (2003)
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rahman, N., Husain, M., Yang, J. et al. First Principle Study of Structural, Electronic, Elastic, and Magnetic Properties of Half-Heusler Compounds ScTiX (X = Si, Ge, Pb, In, Sb, and Tl). J Supercond Nov Magn 33, 3915–3922 (2020). https://doi.org/10.1007/s10948-020-05652-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-020-05652-6