Abstract
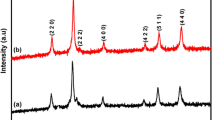
Nanoparticles (NP) of MnFe2 O 4 were synthesized via sol-gel method, with calcination process at 600 °C. The effects of different surfactants namely polypropylene glycol (PPG) and polysorbate 80 (PS80) in the formation of crystal structure, magnetic properties, and optical properties were studied using X-ray diffraction (XRD), vibration sample magnetometry, UV-visible diffuse reflectance spectroscopy (DRS), and photoluminescence (PL) spectroscopy. Brunauer-Emmett-Teller (BET) surface area test was also performed. XRD investigations revealed that MnFe2 O 4 sample synthesized using PS80 as surfactant (sample 2) showed pure crystalline phase of a cubic spinel structure whereas sample synthesized with PPG (sample 1) exhibited an additional phase of α-Fe2 O 3. The morphology of the samples was recorded using a scanning electron microscope (SEM). Energy dispersive X-ray (EDX) results showed that the composition of the elements was as relevant as expected from the sol-gel method. The magnetic hysteresis loops confirmed the super-paramagnetic behavior of both the samples. PL studies revealed a sharp narrow band green emission exhibited by both the samples. Catalytic activity of MnFe2 O 4 nanoparticles (NPs) was performed. The catalysts MnFe2 O 4, samples 1 and 2, were tested for the oxidation of benzyl alcohol to benzaldehyde. The conversion of benzyl alcohol reached a maximum of 82.45 % for MnFe2 O 4 sample 2, whereas for sample 1, the conversion was only 68.52 %. These Mn ferrite samples have potential applications in LED or laser diodes.






Similar content being viewed by others
References
Horvath, M.P. J. Magn. Magn. Mater. 215(171) (2000)
Adam, J.D., Davis, L.E., Dionne, G.F., Schloemann, E.F., Stitzer, S.N.: IEEE Trans. Microwav. Theory Tech. 50, 721 (2002)
Zhou, Z.H., Xue, J.M., Wang, J., Chan, H.S.O., Yu, T., Shen, Z.X.: J. Appl. Phys. 91, 6015 (2002)
Arulmurugan, R., Jeyadevan, B., Vaidyanathan, G., Sendhilnathan, S.: J. Magn. Magn. Mater. 288, 470 (2005)
Fujioka, H., Ikeda, T., Ono, K., Ito, S., Oshima, M.: J. Cryst. Growth 241, 309 (2002)
Salah, L.M., Moustafa, A.M., Farag, I.S.A.: Ceram. Int. 38, 5605 (2012)
Phumying, S., Labuayai, S., Swatsitang, E., Amornkitbamrung, V., Maensiri, S.: Mater. Res. Bull. 48, 2060 (2013)
Guo, P, Zhang, G, Yu, J, Li, H., Zhao, X.S.: Physicochem. Eng. Aspects 395, 168 (2012)
Chen, D., Liu, H., Li, L.: Mater. Chem. Phys. 134, 921 (2012)
Li, J., Yuan, H., Li, G., Liu, Y., Leng, J.: J. Magn. Magnc. Mater. 322, 3396 (2010)
Amighian, J., Mozaffari, M., Nasr, B.: Phys. Status. Solidi C 3, 3188 (2006)
Liu, C, Zou, B, Rondinone, AJ, Zhang, ZJ: J. Phys. Chem. B 104 (2000)
Sivakumar, M., Kanagesan, S., Chinnaraj, K., Sureshbabu, R., Nithiyanantham, S.: J. Inorg. Organomet. Polym. Mater. 23, 439 (2013)
Ramankutty, C.G., Sugunan, S.: Appl. Catal. A Gen. 218, 39 (2001)
Rivas, P., Sagredo, V., Rossi, F., Pernechele, C., Solzi, M., Pena, O.: IEEE Trans. Magn. 49, 4614 (2013)
Li, K., Cheng, R., Wang, S., Zhang, Y.: J. Phys. Condens. Matter. 10, 4315 (1998)
Cannas, C., Ardu, A., Musinu, A., Peddis, D., Piccaluga, G.: Chem. Mater. 20, 6364 (2008)
Brabers, V.A.M.: Phys. Status Solidi B. 33, 563 (1969)
Gharagozlou, M.: J. Alloy. Compd. 486, 660 (2009)
Bhargava, R, Sharma, PK, Dutta, RK, Kumar, S, Pandey, A.C., Kumar, N.: Mater. Chem. Phys. 120, 393 (2010)
Yang, J., Gao, M., Yang, L., Zhang, Y., Lang, J., Wang, D., Wang, Y., Liu, H., Fan, H.: Appl. Surf. Sci. 255, 2646 (2008)
Joy, P.A., Date, S.K.: J. Magn. Magn. Mater. 222, 33 (2000)
Kasapoglu, N., Baykal, A., Koseoglu, Y., Toprak, M.S.: Scr. Mater. 57, 441 (2007)
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Jacintha, M., Neeraja, P., Sivakumar, M. et al. Comparative Study of MnFe2 O 4 Nanoparticles Synthesized by Sol-gel Method with Two Different Surfactants. J Supercond Nov Magn 30, 237–242 (2017). https://doi.org/10.1007/s10948-016-3714-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-016-3714-9