Abstract
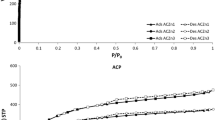
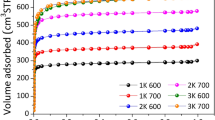
Microporous activated carbons (ACs) derived from biomass residues, by virtue of their low-cost, good thermo-mechanical stability and easy adsorbent regeneration, are widely considered as hydrogen storage materials for near-term applications. The hydrogen uptake performance of activated carbons is known to depend on the pore-textural and surface characteristics, such as size and distribution of micropores and specific surface area. Here, we present a detailed investigation on how the activation processes using KOH, CO2, K2CO3, and H3PO4 modify the microstructure of olive stones-derived ACs and how they affect the ACs’ hydrogen storage behavior. The KOH-activation results in the formation of exfoliated graphene sheets, which are not common in lignocellulose-derived ACs. In addition, the KOH-activation forms supermicropores (1–2 nm) that enhance the hydrogen uptake capacity at high pressures (200 bar). The absolute hydrogen adsorption of KOH-activated sample at 200 bar and 77 K is 6.11 wt%, which is among the highest reported for activated carbon samples. The best hydrogen uptake density per surface area of the carbon we obtained is 2.1 × 10−3 wt% m−2 g which is very close to the theoretical maximum hydrogen uptake density on a single graphene sheet. CO2 and H3PO4 activations are more effective on the creation of ultramicropores (d ≤ 0.7 nm) in the carbon matrix. This order of pore size is useful when hydrogen adsorption is performed at sub-atmospheric pressures. Our study suggests that activated carbons with a homogenous pore size distribution centered at narrow range are not as efficient H2 adsorbents as the ACs with a bimodal PSD.











Similar content being viewed by others
References
B. Panella, M. Hirscher, H. Pütter, U. Müller, Hydrogen adsorption in metal–organic frameworks: Cu-MOFs and Zn-MOFs compared. Adv. Funct. Mater. 16(4), 520–524 (2006)
J.L.C. Rowsell, A.R. Millward, K.S. Park, O.M. Yaghi, Hydrogen sorption in functionalized metal–organic Frameworks. J. Am. Chem. Soc. 126(18), 5666–5667 (2004)
Y. Li, R.T. Yang, Hydrogen storage in low silica type X zeolites. J. Phys. Chem. B 110(34), 17175–17181 (Aug. 2006)
Y. Yürüm, A. Taralp, T.N. Veziroglu, Storage of hydrogen in nanostructured carbon materials. Int. J. Hydrog. Energy 34(9), 3784–3798 (2009)
S.J. Yang, H. Jung, T. Kim, C.R. Park, Recent advances in hydrogen storage technologies based on nanoporous carbon materials. Prog. Nat. Sci. 22(6), 631–638 (2012)
A.J. Kidnay, M.J. Hiza, in Advances in Cryogenic Engineering, ed. K. D. Timmerhaus (Springer, Berlin, 1967), pp. 730–740
C. Carpetis, W. Peschka, A study on hydrogen storage by use of cryoadsorbents. Int. J. Hydrog. Energy 5(5), 539–554 (1980)
R.K. Agarwal, J.S. Noh, J.A. Schwarz, P. Davini, Effect of surface acidity of activated carbon on hydrogen storage. Carbon 25(2), 219–226 (1987)
J.S. Noh, R.K. Agarwal, J.A. Schwarz, Hydrogen storage systems using activated carbon. Int. J. Hydrog. Energy 12(10), 693–700 (1987)
H. Jin, Y.S. Lee, I. Hong, Hydrogen adsorption characteristics of activated carbon. Catal. Today 120(3–4), 399–406 (2007)
M. Zyzlila Figueroa-Torres, A. Robau-Sánchez, L. De la Torre-Sáenz, A. Aguilar-Elguézabal, Hydrogen adsorption by nanostructured carbons synthesized by chemical activation. Microporous Mesoporous Mater. 98(1–3), 89–93 (2007)
H. Akasaka, T. Takahata, I. Toda, H. Ono, S. Ohshio, S. Himeno, T. Kokubu, H. Saitoh, Hydrogen storage ability of porous carbon material fabricated from coffee bean wastes. Int. J. Hydrog. Energy 36(1), 580–585 (2011)
D. Wang, Z. Geng, C. Zhang, X. Zhou, X. Liu, Effects of thermal activation conditions on the microstructure regulation of corncob-derived activated carbon for hydrogen storage J. Energy Chem. 23(5), 601–608 (2014)
A. Minoda, S. Oshima, H. Iki, E. Akiba, Synthesis of KOH-activated porous carbon materials and study of hydrogen adsorption J. Alloys Compd. 580(1), S301–S304 (2013)
J. Wang, I. Senkovska, S. Kaskel, Q. Liu, Chemically activated fungi-based porous carbons for hydrogen storage. Carbon 75, 372–380 (2014)
Y. Xiao, H. Dong, C. Long, M. Zheng, B. Lei, H. Zhang, Y. Liu, Melaleuca bark based porous carbons for hydrogen storage. Int. J. Hydrog. Energy 39(22), 11661–11667 (2014)
I. Wróbel-Iwaniec, N. Díez, G. Gryglewicz, Chitosan-based highly activated carbons for hydrogen storage. Int. J. Hydrog. Energy 40(17), 5788–5796 (2015)
Y.-J. Heo, S.-J. Park, Synthesis of activated carbon derived from rice husks for improving hydrogen storage capacity. J. Ind. Eng. Chem. 31, 330–334 (2015)
N. Texier-Mandoki, J. Dentzer, T. Piquero, S. Saadallah, P. David, C. Vix-Guterl, Hydrogen storage in activated carbon materials: Role of the nanoporous texture. Carbon 42(12–13), 2744–2747 (2004)
Y. Gogotsi, C. Portet, S. Osswald, J.M. Simmons, T. Yildirim, G. Laudisio, J.E. Fischer, Importance of pore size in high-pressure hydrogen storage by porous carbons. Int. J. Hydrog. Energy 34(15), 6314–6319 (2009)
I. Cabria, M.J. López, J.A. Alonso, The optimum average nanopore size for hydrogen storage in carbon nanoporous materials. Carbon 45(13), 2649–2658 (2007)
M. Sevilla, R. Mokaya, A.B. Fuertes, Ultrahigh surface area polypyrrole-based carbons with superior performance for hydrogen storage. Energy Environ. Sci. 4(8), 2930–2936 (2011)
G. Houcine, O. Abdelmottaleb, Transformation du grignon d’olive Tunisien en charbon actif par voie chimique à l’acide phosphorique. Récents Progrés En Génie Procédés 92 (2005)
N. Bader, A. Ouederni, Optimization of biomass-based carbon materials for hydrogen storage. J. Energy Storage 5, 77–84 (2016)
D. Cazorla-Amorós, J. Alcañiz-Monge, M.A. de la Casa-Lillo, A. Linares-Solano, CO2 as an adsorptive to characterize carbon molecular sieves and activated carbons. Langmuir 14(16), 4589–4596 (1998)
Y. Ye, Interaction of hydrogen with novel carbon materials, Ph.D. California Institute of Technology, 2001
M. Molina-Sabio, F. Rodríguez-Reinoso, Role of chemical activation in the development of carbon porosity. Colloids Surf. Physicochem. Eng. Asp. 241(1–3), 15–25 (2004)
M. Jagtoyen, F. Derbyshire, Activated carbons from yellow poplar and white oak by H3PO4 activation. Carbon 36(7–8), 1085–1097 (1998)
F. Rodríguez-Reinoso, J.M. Martín-Martínez, M. Molina-Sabio, I. Pérez-Lledó, C. Prado-Burguete, A comparison of the porous texture of two CO2 activated botanic materials. Carbon 23(1), 19–24 (1985)
P. Ehrburger, A. Addoun, F. Addoun, J.-B. Donnet, Funcat Cogas’86: the international symposium carbonization of coals in the presence of alkaline hydroxides and carbonates: formation of activated carbons. Fuel 65(10), 1447–1449 (1986)
K.S.W. Sing, D.H. Everett, R.A.W. Haul, L. Moscou, R.A. Pierotti, J. Rouquerol, T. Siemieniewska, Reporting physisorption data for gas/solid systems, in: H. Ertl Handbook of Heterogeneous Catalysis, (Wiley, Hoboken, 2008)
H. Wang, Q. Gao, J. Hu, High hydrogen storage capacity of porous carbons prepared by using activated carbon. J. Am. Chem. Soc. 131(20), 7016–7022 (2009)
M. Kunowsky, J.P. Marco-Lozar, A. Oya, A. Linares-Solano, Hydrogen storage in CO2-activated amorphous nanofibers and their monoliths. Carbon 50(3), 1407–1416 (2012)
D. Qu, Investigation of hydrogen physisorption active sites on the surface of porous carbonaceous materials. Chem. Weinh. Bergstr. Ger 14(3), 1040–1046 (2008)
M. Georgakis, G. Stavropoulos, G.P. Sakellaropoulos, Molecular dynamics study of hydrogen adsorption in carbonaceous microporous materials and the effect of oxygen functional groups. Int. J. Hydrog. Energy 32(12), 1999–2004 (2007)
M.J. Bleda-Martínez, J.M. Pérez, A. Linares-Solano, E. Morallón, D. Cazorla-Amorós, Effect of surface chemistry on electrochemical storage of hydrogen in porous carbon materials. Carbon 46(7), 1053–1059 (2008)
R.K. Dash, Nanoporous Carbons Derived from Binary Carbides and their Optimization for Hydrogen Storage, (Drexel University, Philadelphia, 2006)
L. Zhou, Y. Zhou, Y. Sun, Enhanced storage of hydrogen at the temperature of liquid nitrogen. Int. J. Hydrog. Energy 29(3), 319–322 (2004)
M. Kunowsky, B. Weinberger, F. Lamari Darkrim, F. Suárez-García, D. Cazorla-Amorós, A. Linares-Solano, Impact of the carbonisation temperature on the activation of carbon fibres and their application for hydrogen storage. Int. J. Hydrog. Energy 33(12), 3091–3095 (2008)
M. Kunowsky, J.P. Marco-Lozar, D. Cazorla-Amorós, A. Linares-Solano, Scale-up activation of carbon fibres for hydrogen storage. Int. J. Hydrog. Energy 35(6), 2393–2402 (2010)
P. Chen, X. Wu, J. Lin, K.L. Tan, High H2 uptake by alkali-doped carbon nanotubes under ambient pressure and moderate temperatures. Science 285(5424), 91–93 (1999)
Y. Kojima, Y. Kawai, A. Koiwai, N. Suzuki, T. Haga, T. Hioki, K. Tange, Hydrogen adsorption and desorption by carbon materials J. Alloys Compd. 421(1–2), 204–208 (2006)
S.J. Yang, T. Kim, J.H. Im, Y.S. Kim, K. Lee, H. Jung, C.R. Park, MOF-derived hierarchically porous carbon with exceptional porosity and hydrogen storage capacity. Chem. Mater. 24(3), 464–470 (2012)
C. Zhang, Z. Geng, M. Cai, J. Zhang, X. Liu, H. Xin, J. Ma, Microstructure regulation of super activated carbon from biomass source corncob with enhanced hydrogen uptake. Int. J. Hydrog. Energy 38(22), 9243–9250 (2013)
B. Panella, Hydrogen Storage by Physisorption on Porous Materials. (Max-Planck-Institut für Metallforschung, Stuttgart, 2006)
A. Züttel, P. Sudan, P. Mauron, P. Wenger, Model for the hydrogen adsorption on carbon nanostructures. Appl. Phys. A 78(7), 941–946 (2004)
Acknowledgements
The authors gratefully acknowledge Dr. F. Reinoso-Rodriguez (University of Alicante, Spain), Dr. R. Chahine (IRH, Université du Québec à Trois Rivières, Canada), and Dr. M. Hirscher (Max Planck Inst. for Intelligent Systems, Stuttgart, Germany) for accepting the research internship of N. Bader. Special thanks go to the University of Gabes for funding the three internships.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bader, N., Zacharia, R., Abdelmottaleb, O. et al. How the activation process modifies the hydrogen storage behavior of biomass-derived activated carbons. J Porous Mater 25, 221–234 (2018). https://doi.org/10.1007/s10934-017-0436-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-017-0436-8