Abstract
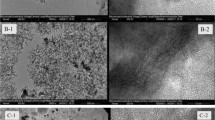
In this work, mesoporous maghemite (γ-Fe2O3) was prepared by the thermal decomposition of [Fe(CON2H4)6](NO3)3 with the aid of cetyltrimethyl ammonium bromide (CTAB), and its adsorption ability for the removal of methyl orange (MO) from wastewater was investigated. X-Ray powder diffraction (XRD) together with nitrogen adsorption–desorption measurements show the formation of mesoporous γ-Fe2O3 with an average pore size of 3.5 nm and a specific surface area of 93.0 m2/g. Magnetic measurements show that the mesoporous γ-Fe2O3 exhibits ferrimagnetic characteristics with the coercivity of 141.5 Oe and remanent magnetization of 7.3 emu/g and has the maximum saturation magnetization of 55.2 emu/g. The adsorption of MO on the mesoporous γ-Fe2O3 reaches the maximum adsorbed percentage of ca. 90% within a few minutes, showing that most of MO can be removed in a short time when the mesoporous γ-Fe2O3 is used as an adsorbent. When the pH of MO solution is varied from 3 to 11, the adsorbed percentage of MO decreases from ca. 90 to ca. 81%, showing that the adsorption is slightly influenced by solution pH. The adsorption data for MO fit well with either Langmuir or Freundlich adsorption models. The maximum adsorption capacity of the mesoporous γ-Fe2O3 for MO is determined to be 385 mg/g, which suggests that the material could be an excellent magnetic adsorbent for MO.









Similar content being viewed by others
References
F. Herrea, A. Lopez, G. Mascolo, P. Albers, J. Kiwi, Appl. Catal. B: Environ. 29, 147 (2001)
Y. Fu, T. Viaraghavan, Bioresource Technol. 79, 251 (2001)
R.Y. Ning, Desalination 143, 237 (2002)
B.V.D. Bruggen, C. Vandecasteele, Environ. Poll. 122, 435 (2003)
V.K. Guptaa, C.K. Jainb, I. Alib, M. Sharmaa, V.K. Saini, Water Res. 37, 4038 (2003)
C.I. Pearcea, J.R. Lloydb, J.T. Guthrie, Dyes Pigments 58, 179 (2003)
S.M. Xu, S. Feng, G. Peng, J.D. Wang, A. Yushan, Carbohyd. Polym. 60, 301 (2005)
S. Yean, L. Cong, C.T. Yavuz, J.T. Mayo, W.W. Yu, A.T. Kan, V.L. Colvin, M.B. Tomson, J. Mater. Res. 20, 3255 (2005)
C. Yavuz, J.T. Mayo, W.W. Yu, A. Prakash, J. Falkner, S. Yean, L. Cong, H. Shipley, A. Kan, M. Tomson, D. Natelson, V.L. Colvin, Science 314, 964 (2006)
J.T. Mayo, C. Yavuz, S. Yean, L. Cong, H. Shipley, W.W. Yu, J. Falkner, A. Kan, M. Tomson, V.L. Colvin, Sci. Technol. Adv. Mater. 8, 71 (2007)
J.S. Hu, L.S. Zhong, W.G. Song, L.J. Wan, Adv. Mater. 20, 2977 (2008)
T. Tuutijärvi, J. Lu, M. Sillanpää, G. Chen, J. Hazard. Mater. 166, 1415 (2009)
R. Wu, J. Qu, H. He, Y. Yu, Appl. Catal. B: Environ. 48, 49 (2004)
R. Wu, J. Qu, Y. Chen, Water Res. 39, 630 (2005)
L.S. Zhong, J.S. Hu, H.P. Liang, A.M. Cao, W.G. Song, L.J. Wan, Adv. Mater. 18, 2426 (2006)
A.D. Ebner, J.A. Ritter, J.P. Harry, Sep. Sci. Technol. 34, 1277 (1999)
G. Reiss, A. Hütten, Nat. Mater. 4, 725 (2005)
J. Cheon, N.-J. Kang, S.-M. Lee, J.-H. Lee, J.-H. Yoon, S.J. Oh, J. Am. Chem. Soc. 126, 1950 (2004)
S. Asuha, S. Zhao, H.Y. Wu, L. Song, O. Tegus, J. Alloys Compd. 472, L23 (2009)
S. Zhao, H.Y. Wu, L. Song, O. Tegus, S. Asuha, J. Mater. Sci. 44, 926 (2009)
Y. Li, W. Tjandra, K.C. Tam, Mater. Res. Bull. 43, 2318 (2008)
D.H. Chen, Y.Y. Chen, J. Colloid Interf. Sci. 236, 41 (2001)
K. Nakajima, M. Hara, K. Domen, J.N. Kondo, Chem. Lett. 34, 394 (2005)
H. Zhao, W. He, Y. Feng, X. Jia, X. Zhang, Z. Li, S. Yan, W. Zhou, Chem. Eng. Technol. 30, 1010 (2007)
D. Huang, Y.J. Wang, Y.C. Cui, G.S. Luo, Micropor. Mesopor. Mater. 116, 378 (2008)
F. Jiao, J.H. Jumas, M. Womes, A.V. Chadwick, A. Harrison, P.G. Bruce, J. Am. Chem. Soc. 128, 12905 (2006)
B. Martinez, X. Obradors, Ll. Balcells, A. Rouanet, C. Monty, Phys. Rev. Lett. 80, 181 (1998)
M.P. Morales, S. Veitemillas-Verdaguer, M.I. Montero, C.J. Sema, A. Roig, Ll. Casas, B. Martínez, F. Sandiumenge, Chem. Mater. 11, 3058 (1999)
R.H. Kodama, A.E. Berkowitz, E.J. McNiff, S. Foner, Phys. Rev. Lett. 77, 394 (1996)
M. Anbia, S.A. Hariri, S.N. Ashrafizadeh, Appl. Surf. Sci. 256, 3228 (2010)
I. Langmuir, J. Am. Chem. Soc. 40, 1361 (1918)
H.M.F. Freundlich, Z. Phys. Chem. 57, 385 (1906)
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (Grant No. 20861006) and Natural Science Foundation of Inner Mongolia (Grant No. MS0806).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Asuha, S., Gao, Y.W., Deligeer, W. et al. Adsorptive removal of methyl orange using mesoporous maghemite. J Porous Mater 18, 581–587 (2011). https://doi.org/10.1007/s10934-010-9412-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-010-9412-2