Abstract
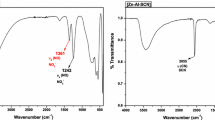
Layered double hydroxide (LDH) is prepared conventionally with bivalent and trivalent cations. We recently reported that the preparation of Zn-Ti LDH consisting of bi and tetravalent cations is possible through the decomposition of urea. In this study, Zn-Mo LDH consisting of bi and hexavalent cations were prepared and reacted with organic monocarboxylic and dicarboxylic acids. Interlayer spacing of the prepared LDH (Zn-Mo-CO3) contained carbonate anions between the layers was 0.72 nm. The spacing was small compared to usual LDH (Zn-Al-CO3) of 0.76 nm in the case of carbonate anion as the guest. Also, TG analysis indicated that the electrostatic force between the Zn-Mo layers and carbonate anions was larger than those of Zn-Al LDH. Certainly, the carbonate anions in Zn-Mo LDH decomposed at 260°C while they in usual LDH decomposed at 230–240°C. ESCA showed that Mo5+ had changed to Mo6+ through the preparation procedure. These results showed the preparation of layered double hydroxides consisting of bivalent and hexavalent cations. By the intercalation of Zn-Mo LDH with suberic acid at 60°C, a sharp peak was observed at 1.06 nm and the peak of LDH itself (0.72 nm) disappeared. This result has suggested that the intercalation of organic acid into new LDH was performed completely.
Similar content being viewed by others
References
F. Cavani, F. Trifiro, A. Vaccari, Catal. Today 11, 173 (1991)
A. Deroy, C. Forano, K. El Malki, J.P. Besse in Anionic Clays: Trends in Pillaring Chemistry, Vol. 2, ed. M.L. Occelli, H.E. Robson (Van Nostrand Reinhold, New York, 1992), p.␣108
E.L. Crepaldi, J.B. Valim, Quim. Nova 21, 300 (1998)
P.C. Pavan, E.L. Crepaldi, G.D. Gomes, J.B. Valim, Colloids Surf., A 154, 399 (1999)
J. Tronto, K.C. Sanchez, E.L. Crepaldi, Z. Naal, S.I. Klein, J.B. Valim, J. Phys. Chem. Solids 65, 493 (2004)
M. Lakraimi, A. Legrouri, A. Barroug, A.D. Roy, J.P. Besse, J. Mater. Chem. 10, 1007 (2000)
S. Velu, K. Suzuki, T. Osaki, F. Ohashi, S. Tomura, Mater. Res. Bull. 34, 1707 (1999)
O. Saber, H. Tagaya, J. Incl. Phenom. Macro. Chem. 45, 109 (2003)
O. Saber, H. Tagaya, J. Porous. Mat. 10, 83 (2003)
M. Meyn, K. Beneke, G. Lagaly, Inorg. Chem. 29, 5201 (1990)
A. Schmassmann, A. Tarnawski, B. Flogerzi, M. Sanner, L.Varga, F. Halter, Eur. J. Gastroenterol. Hepatol. 5, 111 (1993)
O. Saber, H. Tagaya, J. Incl. Phenom. Macro. Chem. 51, 17 (2005)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muramatsu, K., Saber, O. & Tagaya, H. Preparation of new layered double hydroxide, Zn-Mo LDH. J Porous Mater 14, 481–484 (2007). https://doi.org/10.1007/s10934-006-9043-9
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-006-9043-9