Abstract
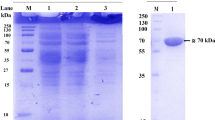
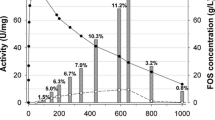
The fructosyltransferase gene was isolated and cloned from Aspergillus oryzae. The gene was 1368 bp, which encoded a protein of 455 amino acids. To analyze the activity of the expressed fructosyltransferase, the pET32a-fructosyltransferase recombined plasmid was transformed into Escherichia coli BL21. The fructosyltransferase gene was successfully expressed by Isopropyl-β-d-thiogalactoside (IPTG) induction. The molecular weight of the expression protein was about 45 kDa. The optimal conditions of protein expression were 25 °C, 0.1 mM IPTG, and 8 h of inducing time. The optimal concentration of urea dealing with inclusion body was 2.5 M. The expressed protein exhibited a strong fructosyl transfer activity. These results showed that the expressed fructosyltransferas owned transferase activity, and could catalyze the synthesis of sucrose-6-acetate.












Similar content being viewed by others
Abbreviations
- A. oryzae :
-
Aspergillus oryzae
- E. coli :
-
Escherichia coli
- IPTG:
-
Isopropyl-β-d-thiogalactoside
- HPLC:
-
High performance liquid chromatography
- FOS:
-
Fructooligosaccrides
- PDA:
-
Potato dextrose agar
- LB:
-
Luria–Bertani
- PCR:
-
Polymerase chain reaction
- SDS-PAGE:
-
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
References
Mutanda T, Mokoena MP, Olaniran AO, Wilhelmi BS, Whiteley CG (2014) Microbial enzymatic production and applications of short-chain fructooligosaccharides and inulooligosaccharides: recent advances and current perspectives. J Ind Microbiol Biotechnol 41(6):893–906
Silva MF, Rigo D, Mossi V, Golunski S, Kuhn Gde O, Di Luccio M, Dallago R, de Oliveira D, Oliveira JV, Treichel H (2013) Enzymatic synthesis of fructooligosaccharides by inulinases from Aspergillus niger and Kluyveromyces marxianus NRRL Y-7571 in aqueous-organic medium. Food Chem 138(1):148–153
Zambelli P, Tamborini L, Cazzamalli S, Pinto A, Arioli S, Balzaretti S, Plou FJ, Fernandez-Arrojo L, Molinari F, Conti P, Romano D (2016) An efficient continuous flow process for the synthesis of a non-conventional mixture of fructooligosaccharides. Food Chem 190:607–613
Seibel J, Moraru R, Gotze S, Buchholz K, Na’amnieh S, Pawlowski A, Hecht HJ (2006) Synthesis of sucrose analogues and the mechanism of action of Bacillus subtilis fructosyltransferase (levansucrase). Carbohydr Res 341(14):2335–2349
Chuankhayan P, Hsieh CY, Huang YC, Hsieh YY, Guan HH, Hsieh YC, Tien YC, Chen CD, Chiang CM, Chen CJ (2010) Crystal structures of Aspergillus japonicus fructosyltransferase complex with donor/acceptor substrates reveal complete subsites in the active site for catalysis. J Biol Chem 285(30):23251–23264
Sprenger N, Bortlik K, Brandt A, Boller T, Wiemken A (1995) Purification, cloning, and functional expression of sucrose:fructan 6-fructosyltransferase, a key enzyme of fructan synthesis in barley. Proc Natl Acad Sci USA 92(25):11652–11656
Yun JW, Choi YJ, Song CH, Song SK (1999) Microbial production of inulo-oligosaccharides by an endoinulinase from Pseudomonas sp. expressed in Escherichia coli. J Biosci Bioeng 87(3):291–295
Hochstrasser U, Luscher M, De Virgilio C, Boller T, Wiemken A (1998) Expression of a functional barley sucrose-fructan 6-fructosyltransferase in the methylotrophic yeast Pichia pastoris. FEBS Lett 440(3):356–360
Trujillo LE, Arrieta JG, Dafhnis F, Garcia J, Valdes J, Tambara Y, Perez M, Hernandez L (2001) Fructo-oligosaccharides production by the Gluconacetobacter diazotrophicus levansucrase expressed in the methylotrophic yeast Pichia pastoris. Enzyme Microb Technol 28(2–3):139–144
Moriyama S, Tanaka H, Uwataki M, Muguruma M, Ohta K (2003) Molecular cloning and characterization of an exoinulinase gene from Aspergillus niger strain 12 and its expression in Pichia pastoris. J Biosci Bioeng 96(4):324–331
Isono N, Tochihara T, Kusnadi Y, Win TT, Watanabe K, Obae K, Ito H, Matsui H (2004) Cloning and heterologous expression of a beta-fructofuranosidase gene from Arthrobacter globiformis IFO 3062, and site-directed mutagenesis of the essential aspartic acid and glutamic acid of the active site. J Biosci Bioeng 97(4):244–249
Kang HK, Seo MY, Seo ES, Kim D, Chung SY, Kimura A, Day DF, Robyt JF (2005) Cloning and expression of levansucrase from Leuconostoc mesenteroides B-512 FMC in Escherichia coli. Biochim Biophys Acta 1727(1):5–15
Angela AF, Clarita OC, Enrique RP, Gladys IC, Jorge NS, Agustín LM (2007) Molecular characterization of sucrose: sucrose 1-fructosyltransferase (1-SST) from Agave tequilana Weber var. azul. Plant Sci 173(4):478–486
Han Y, Chen L, Mao D, Tang L, Guan L (2010) Expression and activity analysis of sucrose:sucrose 1-fructosyltransferase from onion. New Biotechnol 27(4):324–329
Wei JZ, Chatterton NJ, Larson SR (2001) Expression of sucrose: fructan 6-fructosyltransferase (6-SFT) and myoinositol 1-phosphate synthase (MIPS) genes in barley (Hordeum vulgare) leaves. J Plant Physiol 158:635–643
Gao X, She MY, Yin GX, Yu Y, Qiao WH, Du LP, Ye XG (2010) Cloning and characterization of genes coding for fructan biosynthesis enzymes (FBEs) in triticeae plants. Agric Sci China 9(3):313–324
Li HJ, Yang AF, Zhang XC, Gao F, Zhang JR (2007) Improving freezing tolerance of transgenic tobacco expressing sucrose: sucrose 1-fructosyltransferase gene from Lactuca sativa. Plant Cell 89(1):37–48
Kurakake M, Ogawa K, Sugie M, Takemura A, Sugiura K, Komaki T (2008) Two types of beta-fructofuranosidases from Aspergillus oryzae KB. J Agric Food Chem 56(2):591–596
Schiffman SS, Rother KI (2013) Sucralose, a synthetic organochlorine sweetener: overview of biological issues. J Toxicol Environ Health B Crit Rev 16(7):399–451
Jinno S, Nakamura Y, Nagata M, Takahashi T (2014) 1-Kestose consumption during pregnancy and lactation increases the levels of IgA in the milk of lactating mice. Biosci Biotechnol Biochem 78(5):861–866
Szúts A, Szabó-Révész P (2012) Sucrose esters as natural surfactants in drug delivery systems-A mini-review. Int J Pharmaceut 433(1–2):1–9
Kandra L, Wagner GJ (1990) Chlorsulfuron modifies biosynthesis of acyl acid substituents of sucrose esters secreted by tobacco trichomes. Plant Physiol 94(3):906–912
Rastall RA (2010) Functional oligosaccharides: application and manufacture. Annu Rev Food Sci Technol 1:305–339
Kohli DK, Bachhawat AK (2003) CLOURE: clustal output reformatter, a program for reformatting ClustalX/ClustalW outputs for SNP analysis and molecular systematics. Nucleic Acids Res 31(13):3501–3502
Saraswat M, Musante L, Ravida A, Shortt B, Byrne B, Holthofer H (2013) Preparative purification of recombinant proteins: current status and future trends. Biomed Res Int 2013:312709
Esposito D, Chatterjee DK (2006) Enhancement of soluble protein expression through the use of fusion tags. Curr Opin Biotechnol 17(4):353–358
LaVallie ER, McCoy JM (1995) Gene fusion expression systems in Escherichia coli. Curr Opin Biotechnol 6(5):501–506
Singh SM, Panda AK (2005) Solubilization and refolding of bacterial inclusion body proteins. J Biosci Bioeng 99(4):303–310
Sambrook J, Russell D (2008) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Kurakake M, Masumoto R, Kunihiro M, Kamata A, Saita E, Ukita N (2009) Production of Fructo-oligosaccharides by β-Fructofuranosidases from Aspergillus oryzae KB. J Agric Food Chem 58(1):488–492
Acknowledegments
This work was supported by Basic Research Project of Science and Technology Department of Henan province (No. 132102210409), and National Natural Science Foundation of China (No. 31172175). We thank Dr. Ming Li (University of Virginia, USA) for his critical revising and suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Guan, L., Chen, L., Chen, Y. et al. Expression and Activity Analysis of Fructosyltransferase from Aspergillus oryzae . Protein J 36, 352–360 (2017). https://doi.org/10.1007/s10930-017-9725-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-017-9725-y