Abstract
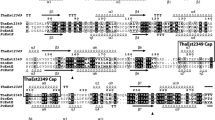
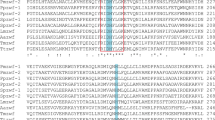
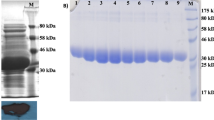
Acetate kinase catalyzes the reversible magnesium-dependent phosphoryl transfer from ATP to acetate to form acetyl phosphate and ADP. Here, we report functional and some structural properties of cold-adapted psychrotrophic enzyme; acetate kinase with those from mesophilic counterpart in Escherichia coli K-12. Recombinant acetate kinase from Shewanella sp. AS-11 (SAK) and E. coli K-12 (EAK) were purified to homogeneity following affinity chromatography and followed by Super Q column chromatography as reported before [44]. Both purified enzymes are shared some of the common properties such as (similar molecular mass, amino acid sequence and similar optimum pH), but characterized shift in the apparent optimum temperature of specific activity to lower temperature as well as by a lower thermal stability compared with EAK. The functional comparisons reveal that SAK is a cold adapted enzyme, having a higher affinity to acetate than EAK. In the acetyl phosphate and ADP-forming direction, the catalytic efficiency (k cat/K m) for acetate was 8.0 times higher for SAK than EAK at 10 °C. The activity ratio of SAK to EAK was increased with decreasing temperature in both of the forward and backward reactions. Furthermore, the activation energy, enthalpy and entropy in both reaction directions that catalyzed by SAK were lower than those catalyzed by EAK. The model structure of SAK showed the significantly reduced numbers of salt bridges and cation-pi interactions as compared with EAK. These results suggest that weakening of intramolecular electrostatic interactions of SAK is involved in a more flexible structure which is likely to be responsible for its cold adaptation.







Similar content being viewed by others
Abbreviations
- EAK:
-
Acetate kinase from Escherichia coli K-12
- SAK:
-
Acetate kinase from Shewanella sp. AS-11
- ES :
-
Enzyme-substrate complex
- ES ‡ :
-
Transition state intermediate
- ΔH ‡ :
-
Activation enthalpy
- ΔS ‡ :
-
Activation entropy
- E a :
-
Activation energy
References
Aceti DJ, Ferry JG (1988) Purification and characterization of acetate kinase from acetate-grown Methanosarcina thermophila: evidence for regulation of synthesis. J Biol Chem 263:15444–15448
Aghajari N, Feller G, Gerday C, Haser R (1988) Structures of the psychrophilic Alteromonas haloplanctis alpha-amylase give insights into cold adaptation at a molecular level. Structure 6:1503–1516
Allen S, Kellermeyer R, Stjernholm R, Wood HJ (1964) Purification and properties of enzymes involved in the propionic acid fermentation. J Bacteriol 87:171–187
Arnold FH, Wintrode PL, Miyazaki K, Gershenson A (2001) How enzymes adapt: lessons from directed evolution. Trends Biochem Sci 26:100–106
Birolo L, Tutino ML, Fontanella B, Gerday C, Mainolfi K, Pascarella S, Sannia G, Vinci F, Marino G (2000) Aspartate aminotransferase from the Antarctic bacterium Pseudoalteromonas haloplanktis TAC 125. Cloning, expression, properties, and molecular modelling. Eur J Biochem 267:2790–2802
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bowman MC, Valdez OR, Nishimura SJ (1976) Acetate kinase from Veillonella alcalescens. J Biol Chem 251:3117–3121
Brown TDK, Jones-Mortimer MC, Kornberg HL (1977) The enzymic interconversion of acetate and acetyl-coenzyme A in Escherichia coli. J Gen Microbiol 102:327–336
Buss KA, Cooper DR, Ingram-Smith C, Ferry JG, Sanders DA, Hasson MS (2001) Urkinase: structure of acetate kinase, a member of the ASKHA superfamily of phosphotransferases. J Bacteriol 183:680–686
Feller G, Gerday C (1997) Psychrophilic enzymes: molecular basis of cold adaptation. Cell Mol Life Sci 53:830–841
Feller G, Arpigny JL, Narinx F, Gerday C (1997) Rethinking immunological privilege: implications for corneal and limbal stem cell transplantation. Comp Biochem Physiol 3:495–499
Feller G, Gerday C (2003) Psychrophilic enzymes: hot topics in cold adaptation. Nat Rev Microbiol 1:200–208
Ferry JG (1992) Biochemistry of methanogenesis. Crit Rev Biochem Mol Biol 27:473–503
Ferry JG (1992) Methane from acetate. J Bacteriol 174:5489–5495
Fields PA, Somero GN (1998) Hot spots in cold adaptation: localized increases in conformational flexibility in lactate dehydrogenase A4 orthologs of Antarctic notothenioid fishes. PNAS 95:11476–11481
Fox DK, Roseman S (1986) Isolation and characterization of homogeneous acetate kinase from Salmonella typhimurium and Escherichia coli. J Biol Chem 261:13487–13497
Gallivan JP, Dougherty DA (1999) Cation-π interactions in structural biology. Biochemistry 96:9459–9464
Gerday C, Aittaleb M, Bentahir M, Chessa JP, Claverie P, Collins T, D’Amico S, Dumont J, Garsoux G, Georlette D, Hoyoux A, Lonhienne T, Meuwis MA, Feller G (2000) Cold-adapted enzymes: from fundamentals to biotechnology. Trends Biotechnol 18:103–107
Gerday C, Aittaleb M, Arpigny JL, Baise E, Chessa JP, Garsoux G, Petrescu I, Feller G (1997) Psychrophilic enzymes: a thermodynamic challenge. Biochim Biophys Acta 1342:119–131
Gerike U, Danson MJ, Russel NJ, Hough DW (1997) Sequencing and expression of the gene encoding a cold-active citrate synthase from an Antarctic bacterium, strain DS2-3R. Eur J Biochem 248:49–57
Gorrell A, Lawrence SH, Ferry JG (2005) Structural and kinetic analyses of arginine residues in the active site of the acetate kinase from Methanosarcina thermophila. J Biol Chem 280:10731–10742
Hochachka PW, Somero GN (1984) Biochemical adaptations. Princeton University Press, Princeton
Iizasa E, Nagano Y (2006) Highly efficient yeast-based in vivo DNA cloning of multiple DNA fragments and the simultaneous construction of yeast/Escherichia coli shuttle vectors. Biotechniques 40:79–83
Ingram-Smith C, Gorrel A, Lawrence SH, Iyer P, Smith K, Ferry JG (2005) Characterization of the acetate binding pocket in the Methanosarcina thermophila acetate kinase. J Bacteriol 187:2386–2394
Knowles JR (1980) Enzyme-catalyzed phosphoryl transfer reactions. Annu Rev Biochem 49:877–919
Kreil-Kiss G, Hoffmann-Ostenhof O (1963) Enzymic formation of adenosine triphosphate with acetyl phosphate as donor in a yeast extract. Biochim Biophys Acta 67:168–170
Kumar S, Nussinov R (1999) Salt bridge stability in monomeric proteins. J Mol Biol 293:1241–1255
Latimer MT, Ferry JG (1993) Cloning, sequence analysis, and hyperexpression of the genes encoding phosphotransacetylase and acetate kinase from Methanosarcina thermophila. J Bacteriol 175:6822–6829
Lipmann F (1944) Enzymatic synthesis of acetyl phosphate. J Biol Chem 155:55–70
Lonhienne T, Mavromatis K, Vorgias CE, Buchon L, Gerday C, Bouriotis VJ (2001) Cloning, sequences, and characterization of two chitinase genes from the Antarctic Arthrobacter sp. strain TAD20: isolation and partial characterization of the enzymes. J Bacteriol 183:1773–1779
Lonhienne T, Zoidakis J, Vorgias CE, Feller G, Gerday C, Bouriotis VJ (2001) Modular structure, local flexibility and cold-activity of a novel chitobiase from a psychrophilic Antarctic bacterium. Mol Biol 310:291–297
Lonhienne T, Gerday C, Feller G (2000) Psychrophilic enzymes: revisiting the thermodynamic parameters of activation may explain local flexibility. Biochim Biophys Acta 1543:1–10
Marshall CJ (1997) Cold-adapted enzymes. Trends Biotechnol 15:359–364
Miyazaki K, Wintrode PL, Grayling RA, Rubingh DN, Arnold FH (2000) Directed evolution study of temperature adaptation in a psychrophilic enzyme. J Mol Biol 297:1015–1026
Morita RY (1975) Psychrophilic bacteria. Bacteriol Rev 39:144–167
Nakajima H, Koichi S, Kazutomo I (1978) Purification and properties of acetate kinase from Bacillus stearothermophilus. J Biochem (Tokyo) 84:193–203
Ohtani N, Haruki M, Morikawa M, Kanaya S (2001) Heat labile ribonuclease HI from a psychrotrophic bacterium: gene cloning, characterization and site-directed mutagenesis. Protein Eng 14:975–982
Purich DL, Fromm HJ (1972) Evaluation of the phosphoryl-enzyme intermediate concept in the acetate kinase and hexokinase reactions from kinetic studies. Arch Biochem Biophys 149:307–315
Rose IA, Grunberg-Manago M, Korey SR, Ochoa S (1954) Enzymatic phosphorylation of acetate. J Biol Chem 211:737–756
Russell NJ (2000) Toward a molecular understanding of cold activity of enzymes from psychrophiles. Extremophiles 4:83–90
Russell RJ, Gerke U, Danson MJ, Hough DW, Taylor GL (1998) Structural adaptations of the cold-active citrate synthase from an Antarctic bacterium. Structure 6:351–361
Sakoda M, Hiromi K (1976) Determination of the best-fit values of kinetic parameters of the Michaelis–Menten equation by the method of least squares with the Taylor expansion. J Biochem 80:547–555
Sali A, Blundell TL (1993) Comparative protein modeling by satisfaction of spatial restraints. J Mol Biol 234:779–815
Tang MAK, Motoshima H, Watanabe K (2012) Cloning, expression, purification of cold adapted acetate kinase from Shewanella species AS-11. Afr J Biotechnol 11:7454–7463
Tang MAK, Motoshima H, Watanabe K (2012) Fluorescence studies on the stability, flexibility and substrate-induced conformational changes of acetate kinases from psychrophilic and mesophilic bacteria. Protein J 31:337–344. doi:10.1007/s10930-012-9408-7
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tang, M.A.K., Motoshima, H. & Watanabe, K. Cold Adaptation: Structural and Functional Characterizations of Psychrophilic and Mesophilic Acetate Kinase. Protein J 33, 313–322 (2014). https://doi.org/10.1007/s10930-014-9562-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-014-9562-1