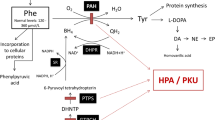
Abstract
Phenylketonuria, the most frequent disorder of amino acid metabolism, is caused by a deficient activity of human phenylalanine hydroxylase (hPAH). Rescue of the enzyme activity of several recombinant hPAH mutant forms (I65T, R261Q, R270K and V388M) by low molecular weight compounds namely glycerol, trimethylamine N-oxide (TMAO) and sodium 4-phenylbutyrate (4-PB) was investigated using a prokaryotic expression model. The studied compounds were added to the culture medium, in a concentration dependent manner, simultaneously to induction of protein expression. Among the tested molecules glycerol and TMAO were able to increase the enzyme activity of the studied mutant proteins. Furthermore, a decrease in aggregates and a recovery of the active tetrameric and dimeric forms were detected. Since the addition of the studied compounds to the medium did not change the expression level of E. Coli molecular chaperones we postulate that glycerol and TMAO rescue results from a direct stabilizing effect of the newly synthesized mutant hPAH enzymes.





Similar content being viewed by others
Abbreviations
- BH4 :
-
(R)-L-erythro-5,6,7,8-tetrahydrobiopterin
- PKU:
-
Phenylketonuria
- HPA:
-
Hyperphenylalaninemia
- MWCO:
-
Molecular weight cut-off
- SEC:
-
Size exclusion chromatography
- HRP:
-
Horseradish peroxidase
References
Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (1994) Current protocols in molecular biology. John Wiley and Sons, New York
Baskakov I, Wang A, Bolen DW (1998) Biophys J 74:2666–2673
Bjorgo E, de Carvalho RM, Flatmark T (2001) Eur J Biochem 268:997–1005
Bolen DW, Baskakov IV (2001) J Mol Biol 310:955–963
Bradford MM (1976) Anal Biochem 72:248–254
Burrows JA, Willis LK, Perlmutter DH (2000) Proc Natl Acad Sci U S A 97:1796–1801
Butler SL, Falke JJ (1996) Biochemistry 35:10595–10600
da Costa MS, Santos H, Galinski EA (1998) Adv Biochem Eng Biotechnol 61:117–153
Davis MD, Parniak MA, Kaufman S, Kempner E (1996) Arch Biochem Biophys 325:235–241
de Almeida SF, Picarote G, Fleming JV, Carmo-Fonseca M, Azevedo JE, de Sousa M (2007) J Biol Chem 282:27905–27912
Gamez A, Perez B, Ugarte M, Desviat LR (2000) J Biol Chem 275:29737–29742
Hansen PA, Waheed A, Corbett JA (2007) Trends Endocrinol Metab 18:1–3
Kaufman S (1987) Methods Enzymol 142:3–17
Knappskog PM, Flatmark T, Aarden JM, Haavik J, Martinez A (1996) Eur J Biochem 242:813–821
Kowlessur D, Citron BA, Kaufman S (1996) Arch Biochem Biophys 333:85–95
Kumar R, Serrette JM, Thompson EB (2005) Arch Biochem Biophys 436:78–82
Kwok SC, Ledley FD, DiLella AG, Robson KJ, Woo SL (1985) Biochemistry 24:556–561
Leandro P, Rivera I, Lechner MC, de Almeida IT, Konecki D (2000) Mol Genet Metab 69:204–212
Leandro P, Lechner MC, Tavares de Almeida I, Konecki D (2001) Mol Genet Metab 73:173–178
Leandro P, Rivera I, Lechner MC, Konecki D, Almeida I (2001) Gene Function and Disease 2:46–50
Martinez A, Knappskog PM, Olafsdottir S, Doskeland AP, Eiken HG, Svebak RM, Bozzini M, Apold J, Flatmark T (1995) Biochem J 306(Pt 2):589–597
Perlmutter DH (2002) Pediatr Res 52:832–836
Pey AL, Stricher F, Serrano L, Martinez A (2007) Am J Hum Genet 81:1006–1024
Pradeep L, Udgaonkar JB (2004) J Biol Chem 279:40303–40313
Rivera I, Cabral A, Almeida M, Leandro P, Carmona C, Eusebio F, Tasso T, Vilarinho L, Martins E, Lechner MC, de Almeida IT, Konecki DS, Lichter-Konecki U (2000) Mol Genet Metab 69:195–203
Saudubray JM, Sedel F, Walter JH (2006) J Inherit Metab Dis 29:261–274
Scriver CR (2007) Hum Mutat 28:831–845
Simon LM, Kotorman M, Garab G, Laczko I (2002) Biochem Biophys Res Commun 293:416–420
Singh LR, Chen X, Kozich V, Kruger WD (2007) Mol Genet Metab 91:335–342
Song JL, Chuang DT (2001) J Biol Chem 276:40241–40246
Stokka AJ, Flatmark T (2003) Biochem J 369:509–518
Uversky VN, Li J, Fink AL (2001) FEBS Lett 509:31–35
Acknowledgements
F. Ventura (iMed.UL) is gratefully acknowledged for critically reading of the manuscript. This work was supported by Fundação para a Ciência e Tecnologia, Portugal, and FEDER (MGI/POCTI/40844/2001, SFRH/BD/10807/2002 and SFRH/BD/19024/2004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nascimento, C., Leandro, J., Tavares de Almeida, I. et al. Modulation of the Activity of Newly Synthesized Human Phenylalanine Hydroxylase Mutant Proteins by Low-Molecular-Weight Compounds. Protein J 27, 392–400 (2008). https://doi.org/10.1007/s10930-008-9149-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-008-9149-9