Abstract
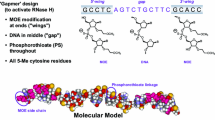
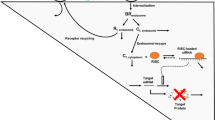
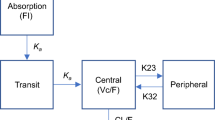
Second-generation antisense oligonucleotides (ASOs) demonstrate excellent biological stability and in vitro/in vivo potency, and thus are considered to be attractive candidates for drugs to treat various diseases. A pharmacokinetic–pharmacodynamic (PK–PD) model of ASOs is desired for the design of appropriate PK and pharmacological studies. The objective of this study was to develop a PK–PD model to accurately simulate hepatic ASO concentration and its efficacy from plasma ASO concentration. After single subcutaneous administration of an ASO targeting hepatic apolipoprotein B (Apo-B) mRNA to mice, the ASO was absorbed rapidly and showed biphasic decline with time from the plasma and liver (t1/2: 1–3 and 81–183 h, Tmax: 0.25–0.50 and 4–8 h). After administration, hepatic Apo-B mRNA and plasma total cholesterol began decreasing at 4–8 and 8–24 h, and their Tmax values were observed at 24–72 and 72 h. To develop the PK–PD model based on the mechanisms of ASOs, we described the plasma and hepatic ASO concentration with linear two-compartment models. In addition, we inserted two indirect response models for mRNA and plasma total cholesterol. Model predictions from plasma ASO concentration gave excellent fits to the observed values of hepatic ASO concentration, Apo-B mRNA and plasma total cholesterol after single or multiple subcutaneous administrations. Our PK–PD model could accurately predict hepatic ASO concentrations and their efficacies from plasma ASO concentrations. This PK–PD model could be a useful tool for suggesting PK and pharmacological study protocols for various liver-targeted second-generation ASOs.





Similar content being viewed by others
References
Yu RZ, Grundy JS, Geary RS (2013) Clinical pharmacokinetics of second generation antisense oligonucleotides. Expert Opin Drug Metab Toxicol 9:169–182. doi:10.1517/17425255.2013.737320
Grünweller A, Hartmann RK (2007) Locked nucleic acid oligonucleotides: the next generation of antisense agents? BioDrugs 21:235–243
Kimber WL, Puri N, Borgmeyer C, Ritter D, Sharov A, Seidman M, Ko MS (2003) Efficacy of 2-methoxyethoxy-modified antisense oligonucleotides for the study of mouse preimplantation development. Reprod Biomed Online 6:318–322
Obika S, Rahman SM, Song B, Onoda M, Koizumi M, Morita K, Imanishi T (2008) Synthesis and properties of 3′-amino-2′,4′-BNA, a bridged nucleic acid with a N3′ → P5′ phosphoramidate linkage. Bioorg Med Chem 16:9230–9237. doi:10.1016/j.bmc.2008.09.013
Straarup EM, Fisker N, Hedtjärn M, Lindholm MW, Rosenbohm C, Aarup V, Hansen HF, Ørum H, Hansen JB, Koch T (2010) Short locked nucleic acid antisense oligonucleotides potently reduce apolipoprotein B mRNA and serum cholesterol in mice and non-human primates. Nucleic Acids Res 38:7100–7111. doi:10.1093/nar/gkq457
Grünweller A, Wyszko E, Bieber B, Jahnel R, Erdmann VA, Kurreck J (2003) Comparison of different antisense strategies in mammalian cells using locked nucleic acids, 2′-O-methyl RNA, phosphorothioates and small interfering RNA. Nucleic Acids Res 31:3185–3193
Geary RS (2009) Antisense oligonucleotide pharmacokinetics and metabolism. Expert Opin Drug Metab Toxicol 5:381–391
Thomas GS, Cromwell WC, Ali S, Chin W, Flaim JD, Davidson M (2013) Mipomersen, an apolipoprotein B synthesis inhibitor, reduces atherogenic lipoproteins in patients with severe hypercholesterolemia at high cardiovascular risk: a randomized, double-blind, placebo-controlled trial. J Am Coll Cardiol 62:2178–2184. doi:10.1016/j.jacc.2013.07.081
Chi KN, Siu LL, Hirte H, Hotte SJ, Knox J, Kollmansberger C, Gleave M, Guns E, Powers J, Walsh W, Tu D, Eisenhauer E (2008) A phase I study of OGX-011, a 2′-methoxyethyl phosphorothioate antisense to clusterin, in combination with docetaxel in patients with advanced cancer. Clin Cancer Res 14(3):833–839
Yu RZ, Lemonidis KM, Graham MJ, Matson JE, Crooke RM, Tribble DL, Wedel MK, Levin AA, Geary RS (2009) Cross-species comparison of in vivo PK/PD relationships for second-generation antisense oligonucleotides targeting apolipoprotein B-100. Biochem Pharmacol 77:910–919. doi:10.1016/j.bcp.2008.11.005
Yu RZ, Zhang H, Geary RS, Graham M, Masarjian L, Lemonidis K, Crooke R, Dean NM, Levin AA (2001) Pharmacokinetics and pharmacodynamics of an antisense phosphorothioate oligonucleotide targeting Fas mRNA in mice. J Pharmacol Exp Ther 296:388–395
Callies S, André V, Patel B, Waters D, Francis P, Burgess M, Lahn M (2011) Integrated analysis of preclinical data to support the design of the first in man study of LY2181308, a second generation antisense oligonucleotide. Br J Clin Pharmacol 71:416–428
Turnpenny P, Rawal J, Schardt T, Lamoratta S, Mueller H, Weber M, Brady K (2011) Quantitation of locked nucleic acid antisense oligonucleotides in mouse tissue using a liquid–liquid extraction LC–MS/MS analytical approach. Bioanalysis 3:1911–1921. doi:10.4155/bio.11.100
Yu RZ, Geary RS, Levin AA (2004) Application of novel quantitative bioanalytical methods for pharmacokinetic and pharmacokinetic/pharmacodynamic assessments of antisense oligonucleotides. Curr Opin Drug Discov Devel 7:195–203
Cen Y, Li X, Liu D, Pan F, Cai Y, Li B, Peng W, Wu C, Jiang W, Zhou H (2012) Development and validation of LC–MS/MS method for the detection and quantification of CpG oligonucleotides 107 (CpG ODN107) and its metabolites in mice plasma. J Pharm Biomed Anal 70:447–455
Ewles M, Goodwin L, Schneider A, Rothhammer-Hampl T (2014) Quantification of oligonucleotides by LC–MS/MS: the challenges of quantifying a phosphorothioate oligonucleotide and multiple metabolites. Bioanalysis 6:447–464
Geary RS, Wancewicz E, Matson J, Pearce M, Siwkowski A, Swayze E, Bennett CF (2009) Effect of dose and plasma concentration on liver uptake and pharmacologic activity of a 2′-methoxyethyl modified chimeric antisense oligonucleotide targeting PTEN. Biochem Pharmacol 78:284–291
Koller E, Vincent TM, Chappell A, De S, Manoharan M, Bennett CF (2011) Mechanisms of single-stranded phosphorothioate modified antisense oligonucleotide accumulation in hepatocytes. Nucleic Acids Res 39:4795–4807
Davies B, Morris T (1993) Physiological parameters in laboratory animals and humans. Pharm Res 10:1093–1095
Lee RG, Crosby J, Baker BF, Graham MJ, Crooke RM (2013) Antisense technology: an emerging platform for cardiovascular disease therapeutics. J Cardiovasc Transl Res 6:969–980. doi:10.1007/s12265-013-9495-7
Prakash TP, Graham MJ, Yu J, Carty R, Low A, Chappell A, Schmidt K, Zhao C, Aghajan M, Murray HF, Riney S, Booten SL, Murray SF, Gaus H, Crosby J, Lima WF, Guo S, Monia BP, Swayze EE, Seth PP (2014) Targeted delivery of antisense oligonucleotides to hepatocytes using triantennary N-acetyl galactosamine improves potency 10-fold in mice. Nucleic Acids Res 42:8796–8807
Dandekar PK, Tessier PR, Williams P, Nightingale CH, Nicolau DP (2003) Pharmacodynamic profile of daptomycin against Enterococcus species and methicillin-resistant Staphylococcus aureus in a murine thigh infection model. J Antimicrob Chemother 52(3):405–411
Pullinger CR, North JD, Teng BB, Rifici VA, Ronhild de Brito AE, Scott J (1989) The apolipoprotein B gene is constitutively expressed in HepG2 cells: regulation of secretion by oleic acid, albumin, and insulin, and measurement of the mRNA half-life. J Lipid Res 30(7):1065–1077
Acknowledgments
The authors would like to thank Ms. Keiko Ogawa, Mr. Takanori Hironaka, Dr. Tatsuya Ikehara, Dr. Ken-ichi Nezasa, Mr. Shingo Sakamoto, and Dr. Shuichi Onishi (Drug Metabolism and Pharmacokinetics, Drug Developmental Research Laboratories, Shionogi & Co., Ltd.) for their support during and discussion related to the animal experiments.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shimizu, R., Kitade, M., Kobayashi, T. et al. Pharmacokinetic–pharmacodynamic modeling for reduction of hepatic apolipoprotein B mRNA and plasma total cholesterol after administration of antisense oligonucleotide in mice. J Pharmacokinet Pharmacodyn 42, 67–77 (2015). https://doi.org/10.1007/s10928-014-9398-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-014-9398-5