Abstract
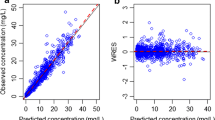
The objectives were to develop a population model for placebo-corrected moxifloxacin QT interval in healthy subjects using non-linear mixed effects modeling and to examine effect of covariates on the observed QT. Based on the parameters of interest, optimizations of observation times and number of subjects were proposed. A pool of four thorough QT studies was used, representing 99 subjects receiving placebo and moxifloxacin. The data was modeled using Monolix. The placebo effect on QT was satisfactorily described using a 2-oscillator model. It reflected the circadian rhythm variability which is taken into account when assessing the time-matched mean difference on QT between treatment and baseline. Based on this model, the moxifloxacin effect on QT was satisfactorily described by the same equation with the adjunct of a direct and proportional drug concentration-effect. The Emax model provided the best description of the effect. The unique covariate was gender for both baseline QTc and individual heart rate correction factor. The present design included up to 16 observations for pharmacodynamics. Using this model, 9 observation times for pharmacodynamics provided satisfactory estimates for the parameters of interest (Emax). With 15% precision limit on Emax, 60 subjects was optimal. The simultaneous placebo-moxifloxacin QT model proposed is an interesting alternative to the ICH E14 guideline in assessing QT prolongation effect. This approach provides accurate information over a range of concentrations using different relationships (slope or Emax models) to quantify the drug-response relationship versus placebo. This allowed optimizing the observation times and number of subjects.




Similar content being viewed by others
References
U.S. Department of Health and Human Services Food and Drug Administration (2005) International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. The clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs (ICH E14). Rockville, MD
Bayer Pharmaceuticals (2003) Avelox (moxifloxacin) package insert. West Haven, CT
Garnett C, Beasley N, Bhattaram VA, Jadhav PR, Madabushi R, Stockbridge N, Tornoe CW, Wang Y, Zhu H, Gobburu JV (2008) Concentration-QT relationships play a key role in the evaluation of proarrhythmic risk during regulatory review. J Clin Pharmacol 48:13–18
Russell T, Riley SP, Cook JA, Richard L, Lalonde RL (2008) A perspective on the use of concentration-QT modeling in drug development. J Clin Pharmacol 48:9–12
Bloomfield D, Krishna R (2008) Commentary on the clinical relevance of concentration/QTc relationships for new drug candidates. J Clin Pharmacol 48:6–8
Grosjean P, Urien S (2012) Reevaluation of moxifloxacin pharmacokinetics and their direct effect on the QT interval. J Clin Pharmacol 52:329–338
Drici MD (2001) Influence of gender on drug-acquired long QT syndrome. Eur Heart J Suppl K(3 Suppl):K41–K47
Stass H, Kubitza D (1999) Pharmacokinetics and elimination of moxifloxacin after oral and intravenous administration in man. J Antimicrob Chemother 43(Suppl B):83–90
Piotrovsky V (2005) Pharmacokinetic-pharmacodynamic modeling in the data analysis and interpretation of drug-induced QT/QTc prolongation. AAPS J 7:E609–E624
Smetana P, Batchvarov V, Hnatkova K, Camm AJ, Malik M (2003) Circadian rhythm of the corrected QT interval: impact of different heart rate correction models. Pacing Clin Electrophysiol 26:383–386
Browne KF, Prystowsky E, Heger JJ, Chilson DA, Zipes DP (1983) Prolongation of the Q-T interval in man during sleep. Am J Cardiol 52:55–59
Malik M, Hnatkova K, Schmidt A, Smetana P (2008) Accurately measured and properly heart-rate corrected QTc intervals show little daytime variability. Heart Rhythm 5(10):1424–1431
Nelson W, Tong YL, Lee JK, Halberg F (1979) Methods for cosinor-rhythmometry. Chronobiologia 6:305–323
Fernandez JR, Hermida RC, Mojo A (2009) Chronobiological analysis techniques. Application to blood pressure. Philos Trans R Soc A 367:431–445
Kuhn E, Lavielle M (2005) Maximum likelihood estimation in nonlinear mixed effects models. Comput Stat Data Anal 49:1020–1030
R Development Core Team (2009) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Savic RA, Jonker DM, Kerbusch T, Karlsson MO (2007) Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn 34:711–726
Sullivan TJ, Lettieri TJ, Liu P, Heller A (2001) The influence of age and gender on the pharmacokinetics of moxifloxacin. Clin Pharmacokinet 40(Suppl 1):1–18
Bazzoli C, Retout S, Mentré F (2010) Design evaluation and optimisation in multiple response nonlinear mixed effect models: PFIM 3.0. Comput Methods Programs Biomed 98(1):55–65
Malik M, Hnatkova K, Ford J, Madge D (2008) Near-thorough QT study as part of a first-in-man study. J Clin Pharmacol 48:1146–1157
Bloomfield DM, Kost JT, Ghosh K, Hreniuk D, Hickey LA, Guitierrez MJ, Gottesdiener K, Wagner JA (2008) The effect of moxifloxacin on QTc and implications for the design of thorough QT studies. Clin Pharmacol Ther 84(4):475–480
Rohatagi S, Carrothers TJ, Kuwabara-Wagg J, Khariton T (2009) Is a thorough QTc study necessary? The role of modeling and simulation in evaluating the QTc prolongation potential of drugs. J Clin Pharmacol 49(11):1284–1296
Acknowledgments
We acknowledge Franck Poitiers, Biostatistics Department of sanofi R&D, for the building of the pooled study database.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
See Table 11.
Rights and permissions
About this article
Cite this article
Grosjean, P., Urien, S. Moxifloxacin versus placebo modeling of the QT interval. J Pharmacokinet Pharmacodyn 39, 205–215 (2012). https://doi.org/10.1007/s10928-012-9242-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-012-9242-8