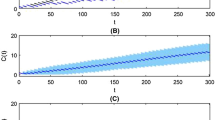
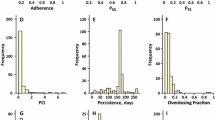
The adherence phenomenon is now well recognized to seriously compromise drug efficacy. In this paper, we analyze the role of compliance through drug intake history as an integral part of the pharmacokinetic process. Being concerned with what is accessible in medical practice, we develop a stochastic approach to model the drug intake behavior that we combine with a conventional pharmacokinetic model in order to investigate the effect of drug intake history on the pharmacokinetic time-course. For this purpose, we explicitly formalize the plasma concentration variations for the most common administration routes. This analytical approach allows to characterize drug concentration variations directly inherited from patient compliance.
Similar content being viewed by others
References
World Health Organization. Adherence to Long-term therapies: Evidence for action. http://www.who.int/chronic_conditions/adherencereport/en/ (2003).
Iskedjian M., Einarson T.R., MacKeigan L.D., Shear N., Addis A., Mittmann N., Ilersich A.L. (2002). Relationship between daily dose frequency and adherence to antihypertensive pharmacotherapy: evidence from a meta-analysis. Clin. Ther. 24(2):302–316
Mu S., Ludden T.M. (2003). Estimation of population harmacokinetic parameters in the presence of non-compliance. JPKPD, 30(1):53–81
Vrijens B., Urquhart J. (2005). Patient adherence to prescribed antimicrobial drug dosing regimens. J. Antimicrob. Chemother. 55:616–627
J. Urquhart. A call for a new discipline. Pharm. Tech. 11(12):617 (Editorial), December (1987).
Vrijens B., Urquhart J. (2005). New findings about patient adherence to prescribed drug dosing regimens: an introduction to pharmionics. Eur. J. Hosp. Pharm. Sci. 11(5):103–106
Gibaldi M., Perrier D. (1982). Pharmacokinetics, 2nd edn. Marcel Dekker, New York, NY
Rowland M., Tozer T.N. (1995). Clinical Pharmacokinetics – Concepts and Applications, 3rd edn. Lea & Febiger, Williams & Wilkins, PA
Advance Analytical Research on Drug Exposure. http://www.aardex.ch/aardex/index.htm.
Vrijens B., Goetghebeur E. (2004). Electronic monitoring of variation in drug intakes can reduce bias and improve precision in pharmacokinetic/pharmacodynamic population studies. Stat. Med. 23:531–544
Labaune J.P. (1988). Pharmacocinétique. Masson, Paris
W. A. Ritschel and G. A. Kearns. Handbook of Basic Pharmacokinetics, 6th edn. APHA (1999).
Girard P., Blaschke T.F., Kastrissios H., Sheiner L.B. (1998). A Markov mixed effect regression model for drug compliance. Stat. Med. 17:2313–2333
Meyer U.A., Peck C.C. (1997). The drug holiday pattern of noncompliance in clinical trials: challenge to conventional concepts of drug safety and efficacy. Center for Drug Development Science. Georgetown University, Washington, DC
Ng P.K. (1981). Prediction of multiple-dose blood level curves of drugs administered four times daily at non-uniform dosing intervals. Int. J. Biomed. Comput. 12:217–226
Faed E.M. (1981). Model-independent linear pharm,acokinetic equations for variable dosing regimen. Biopharm Drug Dispos. 2:299–302
Niebergall P.J., Sugita E.T., Schnaare R.L. (1974). Calculation of plasma level versus time profiles for variable dosing regimens. J. Pharm. Sci. 63:100–105
Howell J.R. (1975). Mathematical formulation for nonuniform multiple dosing. J. Pharm. Sci. 64:464–466
J. A. Jacquez. Compartmental Analysis in Biology and Medicine, 3rd edn. Biomedware (1996).
C. E. Petit-jetté, J. Li, J. del Castillo, and F. Nekka. Influence of different feeding behavior scenarios on the pharmacokinetics of feed-administered antibiotics in swine: application to chlortetracycline. Submitted.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, J., Nekka, F. A Pharmacokinetic Formalism Explicitly Integrating the Patient Drug Compliance. J Pharmacokinet Pharmacodyn 34, 115–139 (2007). https://doi.org/10.1007/s10928-006-9036-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-006-9036-y