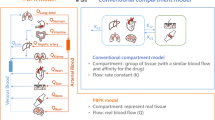
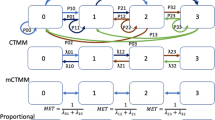
Abstract
To help identify the role of modelling and simulation in the development of anti-cancer agents, their main advantages and the obstacles to their rational use, an expert meeting was organized by COST B15. This manuscript presents a synthesis of views expressed at that meeting and indicates future directions. The manuscript also shows some examples where modelling and simulation have proven to be of relevant value in the drug development process for anti-cancer agents.
Similar content being viewed by others
References
L. Aarons M. O. Karlsson F. Mentré et al. (2001) ArticleTitleRole of modelling and simulation in phase I drug development Eur. J. Pharm. Sci 13 115–122
P. Nygren (2001) ArticleTitleWhat is cancer chemotherapy Acta Oncol 40 166–174 Occurrence Handle11441929
P. Nygren B. Glimelius (2001) ArticleTitleThe Swedish Council on Technology Assessment in Health Care (SBU) report on Cancer Chemotherapy–Project objectives, the working process, key definitions and general aspects on cancer trial methodology and interpretation Acta Oncol 40 155–165 Occurrence Handle11441928
G. Avvisati F. Lo -Coco F. Mandelli (2001) ArticleTitleAcute promyelocytic Leukemia: clinical and morphologic features and prognostic factors Semin. Hematol 38 IssueID1 4–12
E. Buchdunger A. Matter B. J. Druker (2001) ArticleTitleBcr-abl inhibition as a modality of CML therapeutics Biochem. Biophys. Acta 1551 M11–M18 Occurrence Handle11553417
B. Vogelstein E. R. Fearon S. R. Hamilton et al. (1988) ArticleTitleGenetic alterations during colorectal-tumor development N. Engl. J. Med 319 525–532 Occurrence Handle2841597
J. M. Brown A. J. Giaccio (1998) ArticleTitleThe unique physiology of solid tumors: Opportunities (and problems) for cancer therapy Cancer Res 58 1408–1416 Occurrence Handle9537241
R. K. Jain (1998) ArticleTitleDelivery of molecular and cellular medicine to solid tumors J. Control. Release 53 49–67 Occurrence Handle9741913
K. D. Miller C. J. Sweeney G. W. Sledge (2001) ArticleTitleRedefining the target: Chemotherapeutics as antiangiogenics J. Clin. Oncol 19 IssueID4 1195–1206 Occurrence Handle11181686
I. J. Fidler (1999) ArticleTitleCritical determinants of cancer metastasis: Rationale for therapy Cancer Chemother. Pharmacol 43 S3–S10 Occurrence Handle10357552
A. R. Nelson B. Fingleton M. L. Rothenberg M. Matrisian (2000) ArticleTitleMatrix metalloproteinases: biologic activity and clinical implications J. Clin. Oncol 18 IssueID5 1135–1149 Occurrence Handle10694567
E. A. Lovejoy A. R. Clarke D. J. Harrrison (1997) ArticleTitleAnimal models and the molecular pathology of cancer J. Pathol 181 IssueID2 130–135 Occurrence Handle9120714
R. S. Kerbel. What is the optimal rodent model for anti-tumor drug testing? Cancer Metastasis Rev. 17(3):301–304 (1998–1999)
T. Corbett F. Valeriote P. LoRusso et al. (1997) In vivo methods for screening and preclinical testing B. A. Teicher (Eds) Anti-cancer Drug Development Guide Part II Humana Press Totowa, NJ 75–100
J. Yang A. Liu C. Dougherty et al. (2000) ArticleTitleBeware of contaminating mouse cells in human xenografts from nude mice Anti-cancer Res 20 IssueID3A 1635–1639
S. Venkatesh R. A. Lipper (2000) ArticleTitleRole of the development scientist in compound lead selection and optimisation J. Pharm. Sci 89 145–154 Occurrence Handle10688744
L. M. Weisenthal (1992) ArticleTitleAntineoplastic drug screening belongs in the laboratory, not in the clinic J. Natl. Cancer Inst 84 466–469 Occurrence Handle1545430
E. J. Freireich et al. (1965) ArticleTitleQuantitative comparison of toxicity of anti-cancer agents in mouse, rat, hamster, dog, monkey and man Cancer Chemother Rep 50 IssueID4 219–244
A. M. Guarino et al. (1979) ArticleTitleAdequacies and inadequacies in assessing murine toxicity data with antineoplastic agents Cancer Res 39 2204–2210 Occurrence Handle445419
S. S. Burtles D. R. Newell R. E. Henrar T. A. Connors et al. (1995) ArticleTitleRevisions of general guidelines for the preclinical toxicology of new cytotoxic anti-cancer agents in Europe. The Cancer Research Campaign (CRC) Phase I/II Clinical Trials Committee and the European Organization for Research and Treatment of Cancer (EORTC) New Drug Development Office Eur. J. Cancer 31 IssueID3 408–410
J. J. De George A. Chang-Ho P. A. Andrews et al. (1998) ArticleTitleRegulatory considerations for preclinical development of anti-cancer drugs Cancer Chemother. Pharmacol 41 173–185 Occurrence Handle9443633
D. R. Budman A. Calabro W. Kreis (1998) ArticleTitleIn vitro evaluation of synergism or antagonism with combinations of new cytotoxic agents Anti-cancer Drugs 8 697–702
P. Piedbois M. Buyse (1993) ArticleTitleWhat can we learn from a meta-analysis of trials testing the modulation of 5FU by leucovorin? Advanced Colorectal Meta-analysis project Ann. Oncol 4 IssueIDS2 15–19
D. J. Slamon B. Leyland-Jones S. Shak et al. (2001) ArticleTitleUse of chemotherapy plus monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2 N. Engl. J. Med 344 783–792 Occurrence Handle11248153
D. D. Von Hoff (1998) ArticleTitleThere are no bad anti-cancer agents, only bad clinical trial designs – Twenty first Richard and Hilda Rosenthal Foundation Award Lecture Clin. Cancer Res 4 IssueID5 1079–1086 Occurrence Handle9607564
E. L. Korn S. G. Arbuck J. Pluda et al. (2001) ArticleTitleClinical trial designs for cytostatic agents: Are new approaches needed? J. Clin. Oncol 19 IssueID1 265–272 Occurrence Handle11134222
J. A. Sandberg V. P. Parker K. S. Blanchard et al. (2000) ArticleTitlePharmacokinetics and tolerability of an antiangiogenic ribozyme (ANGIOZYME) in healthy volunteers J. Clin. Pharmacol 40 IssueID12 pt 2 1462–1469 Occurrence Handle11185667
M. Buyse P. Piedbois (1996) ArticleTitleOn the relationship between response to treatment and survival time Stat. Med 15 IssueID24 2797–2812 Occurrence Handle8981687
M. Buyse P. Thirion R. W. Carlson et al. (2000) ArticleTitleRelation between tumor response to first line chemotherapy and survival in advanced colorectal cancer: Meta-analysis Group in Cancer Lancet 356 IssueID9227 373–378 Occurrence Handle10972369
InstitutionalAuthorNameAnomymous (1997) ArticleTitleInterferon alfa versus chemotherapy for chronic myeloid leukemia: a meta-analysis of seven randomized trials: Chronic Myeloid leukemia trialists’ Collaborative group J. Natl. Cancer Inst 89 IssueID21 1616–1620
M. J. Mauro M. O’Dwyer M. C. Heinrich B. J. Druker (2001) ArticleTitleSTI571: A paradigm of new agents for cancer therapeutics J. Clin. Oncol 20 325–334
D. L. Toppmeyer (1996) Phase I trial design and methodology B. Teicher (Eds) Anti-cancer Drug Development Guide: Preclinical Screening, Clinical Trials and Approval Humana Press Totowa, NJ 227–247
E. Rowinsky J. J. Windle D. Von Hoff (1999) ArticleTitleRas protein farnesylation. A strategic target for anti-cancer therapeutic Development J. Clin. Oncol 17 IssueID11 3631–3652 Occurrence Handle10550163
N. J. Bundred (2000) ArticleTitlePrognostic and predictive factors in breast cancer Cancer Treat Rev 27 137–142
R. J. Erb (1993) ArticleTitleIntroduction to back propagation neural network computation Pharm Res 10 165–170 Occurrence Handle8456062
R. Simon B. Freidlin L. Rubinstein et al. (1997) ArticleTitleAccelerated titration designs for phase I clinical trials in oncology J. Natl. Cancer Inst 89 1138–1147 Occurrence Handle9262252
J. O’Quigley L. Z. Shen (1996) ArticleTitleContinual reassment method: A likelihood approach Biometrics 52 673–684 Occurrence Handle8672707
T. T. Chen J. P. Chute E. Feigal B. E. Johnson R. Simon (2000) ArticleTitleA model to select chemotherapy regimens for phase III trials for extensive-stage small cell lung cancer J. Natl. Cancer Inst 92 1601–1607 Occurrence Handle11018096
B. Gunnars P. Nygren B. Glimelius (2001) ArticleTitleAssessment of quality of life during chemotherapy Acta Oncol 40 175–184 Occurrence Handle11441930
B. F. Cole R. D. Gelber J. M. Kirkwood et al. (1996) ArticleTitleQuality of life adjusted survival analysis of interferon alfa-2b adjuvant treatment of high-risk resected cutaneous melanoma: An Eastern cooperative oncology group study J. Clin. Oncol 14 2666–2673 Occurrence Handle8874325
R. D. Gelber A. Goldhirsch B. F. Cole et al. (1996) ArticleTitleA quality-adjusted time without symptoms or toxicity (Q-TWIST) analysis of adjuvant radiation therapy and chemotherapy for resectable rectal cancer J. Natl. Cancer Inst 88 1039–1045 Occurrence Handle8683634
V. Levy R. Porcher F. Delabarre M. Leporrier B. Cazin S. Chevret (2001) ArticleTitleEvaluating treatment strategies in chronic lymphocytic leukemia: Use of quality-adjusted survival analysis J. Clin. Epidemiol 54 747–754 Occurrence Handle11438417
M. J. Ratain (1998) ArticleTitleBody-surface area as a basis for dosing of anti-cancer agents: Science, myth or habit J. Clin. Oncol 16 2297–2298 Occurrence Handle9667242
M. Sandström A. Freijs R. Larsson M. P. N. Karlsson J. Bergh (1996) ArticleTitleLack of relationship between systemic exposure for the component drugs of the fluorouracil, epirubicin and 4-hydroxocyclophosphamide regimen in breast cancer patients J. Clin. Oncol 14 1581–1588 Occurrence Handle8622075
H. Gurney (1996) ArticleTitleDose calculation of anti-cancer drugs: A review of the current practice and introduction of an alternative J. Clin. Oncol 14 2590–2611 Occurrence Handle8823340
A. Nirenberg C. Mosende B. M. Mehta A. L. Gisolfi G. Rosen (1977) ArticleTitleHigh-dose methotrexate with citrovorum factor rescue: predictive value of serum methotrexate concentrations and corrective measures to avert toxicity Cancer Treat Rep 61 779–783 Occurrence Handle302143
S. P. Dix J. R. Wingard R. E. Mullins et al. (1996) ArticleTitleAssociation of busulfan area under the curve with veno-occlusive disease following BMT Bone Marrow Transplant 17 225–230 Occurrence Handle8640171
R. Bruno D. Hille A. Riva et al. (1998) ArticleTitlePopulation pharmacokinetics/pharmacodynamics of docetaxel in phase II studies in patients with cancer J. Clin. Oncol 16 187–196 Occurrence Handle9440742
A. H. Calvert D. R. Newell L. A. Gumbrell et al. (1989) ArticleTitleCarboplatin dosage: prospective evaluation of a simple formula based on renal function J. Clin. Oncol 7 1748–1756 Occurrence Handle2681557
M. V. Relling M. L. Hancock G. K. Rivera et al. (1999) ArticleTitleMercaptopurine therapy intolerance and heterozygosity at the thiopurine S-methyltransferase gene locus J. Natl. Cancer Inst 91 2001–2008 Occurrence Handle10580024
G. N. Hortobagyi (2001) ArticleTitleOverview of treatment results with trastuzumab (Herceptin) in metastatic breast cancer Semin. Oncol 28 IssueID6 Suppl 18 43–47
A. R. Ahmed S. M. Hombal (1984) ArticleTitleCyclophosphamide (Cytoxan) a review on relevant pharmacology and clinical uses J. Am. Acad Dermatol 11 1115–1126 Occurrence Handle6392368
J. J. Pérez-Ruixo, V. G. Casabò, M. Merino, et al. Population Pharmacokinetic and Pharmacodynamic of Cyclosphosphamide in Breast Cancer. Population Approach Group Europe 2000. Salamanca http://www.page-meeting.org
R. S. Herbst F. R. Khuri (2003) ArticleTitleMode of action of docetaxel – a basis for combination with novel anticancer agents Cancer Treat Rev 29 407–415 Occurrence Handle12972359
R. Bruno A. Riva D. Hille A. Lebecq L. Thomas (1997) ArticleTitlePharmacokinetic and pharmacodynamic properties of docetaxel, results of phase I and phase II trials Am. J. Health Syst. Pharm 54 IssueID24 Suppl 2 S16–S19 Occurrence Handle9435928
C. Veyrat-Follet, R. Bruno, R. Olivares, G. R. Rhodes, and P. Chaikin. Clinical trial simulation of docetaxel in patients with cancer as a tool for dosage optimization. Clin. Pharmacol. Ther. 68(6):677–687 (2000)
A. J. Wagstaff T. Ibbotson K. L. Goa (2003) ArticleTitleCapecitabine: A review of its pharmacology and therapeutic efficacy in the management of advanced breast cancer Drugs 63 217–236 Occurrence Handle12515569
M. Miwa. M. Ura M. Nishida et al. (1998) ArticleTitleDesign of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumors by enzymes concentrated in human liver and tumor tissue Eur. J. Cancer 34 1274–1281 Occurrence Handle9849491
R. Gieschke, J. L. Steimer and B. G. Reigner. Relationships between metrics of exposure to XelodaTM and occurrence of adverse effects. Annual meeting of the American Society of Clinical Oncology (ASCO), Los Angeles, May 16–19, 1998
R. Gieschke B. G. Reigner J. L. Steimer (1997) ArticleTitleExploring clinical study design by computer simulation based on pharmacokinetic/pharmacodynamic modelling Int. J. Clin. Pharmacol. Ther 35 469–474 Occurrence Handle9352398
R. Gieschke B. G. Reigner K. S. Blesch J. L. Steimer (2002) ArticleTitlePopulation pharmacokinetic analysis of the major metabolites of capecitabine J. Pharmacokin. Pharmacodyn 29 25–47
R. Gieschke H. U. Burger B. G. Reigner K. S. Blesch J. L. Steimer (2003) ArticleTitlePopulation pharmacokinetics and concentration–effect relationships of capecitabine metabolites in colorectal cancer patients Br. J. Clin. Pharmacol 55 252–263 Occurrence Handle12630975
Y. Tsukamoto Y. Kato M. Ura et al. (2001) ArticleTitleA physiologically based pharmacokinetic analysis of capecitabine, a triple Prodrug of 5-FU, in humans: The mechanism for tumor-selective accumulation of 5-FU Pharm Res 18 1190–1202 Occurrence Handle11587492
J. Bennouna P. Fumoleau J. P. Armand et al. (2003) ArticleTitlePhase I and pharmacokinetic study of the new vinca-alkaloid vinflunine administered as a 10 minute infusion every 3 weeks in patients with advanced solid tumors Ann.Oncol 14 IssueID3 630–637 Occurrence Handle12649112
L. Friberg A. Henningsson H. Maas L. Nguyen M. Karlsson (2002) ArticleTitleModel of chemotherapy-induced myelosuppression with parameter consistency across drugs J. Clin. Oncol 20 4713–4721 Occurrence Handle12488418
P. H. Vander Graaf E. A. Van Schaick R. A. A. Mathôt A. P. Ijzerman M. Danhof (1997) ArticleTitleMechanism-based pharmacokinetic–pharmacodynamic modelling of the effects of N6-Cyclopentyladenosine analogs on heart rate in rat: estimation of in vivo operational affinity and efficacy at adenosine A1 receptors J. Pharmacol. Exp. Ther 283 809–816 Occurrence Handle9353402
H. J. Kuh S. H. Jang M. G. Wientjes J. L. S. Au (2000) ArticleTitleComputational model of intracellular pharmacokinetics of paclitaxel J. Pharmacol. Exp. Ther 293 761–770 Occurrence Handle10869374
P. Moine J. X. Mazoit (1999) ArticleTitleStreptococcus pneumoniae pneumonia in mice: Optimal amoxicillin dosing predicted from a pharmacokinetic–pharmacodynamic model J. Pharmacol. Exp. Ther 291 1086–1092 Occurrence Handle10565828
M. Tsuchihashi H. Harashima H. Kiwada (1999) ArticleTitleDevelopment of a pharmacokinetic/pharmacodynamic (pk/pd)-simulation system for doxorubicin in long circulating liposomes in mice using peritoneal P388 J. Control Release 61 9–19 Occurrence Handle10469899
J. O’Quigley M. Pepe L. Fisher (1990) ArticleTitleContinual reassessment method: A practical design for phase I clinical trials in cancer Biometrics 46 33–48 Occurrence Handle2350571
S. Goodman M. Zahurak S. Piantadosi (1995) ArticleTitleSome practical improvements in the continual reassessment method for phase I studies Stat. Med 14 1149–1161 Occurrence Handle7667557
J. O’Quigley (2002) ArticleTitleContinual reassessment designs with early termination Biostatistics 3 87–99 Occurrence Handle12933626
M. Gasparini J. Eisele (2000) ArticleTitleA curve-free method for phase I clinical trials Biometrics 56 609–615 Occurrence Handle10877324 Occurrence HandleMR1795024
P. F. Thall K. E. Russell (1998) ArticleTitleA strategy for dose-finding and safety monitoring based on efficacy and adverse outcomes in phase I/II clinical trials Biometrics 54 251–264 Occurrence Handle9544520
S. Piantadosi G. Liu (1996) ArticleTitleImproved designs for dose escalation studies using pharmacokinetic measurements Stat. Med 15 1605–1618 Occurrence Handle8858785
S. Chevret (1993) ArticleTitleThe continual reassessment method in cancer phase I clinical trials: a simulation study Stat. Med 12 1093–1108 Occurrence Handle8210815
L. Mariani E. Marubini (2000) ArticleTitleContent and quality of currently published phase II cancer trials J. Clin. Oncol 18 429–436 Occurrence Handle10637259
R. Simon (1989) ArticleTitleOptimal two-stage designs for phase II clinical trials Control Clin. Trials 10 1–10 Occurrence Handle2702835
E. A. Gehan (1961) ArticleTitleThe determination of the number of patients required in a preliminary and a follow-up trial of a new chemotherapeutic agent J. Chron. Dis 13 346–353 Occurrence Handle13704181
J. E. Herndon (1998) ArticleTitleA design alternative for two-stage, phase II, multicenter cancer clinical trials Control Clin. Trials 19 440–450 Occurrence Handle9741865
M. Posch P. Bauer (2000) ArticleTitleInterim analysis and sample size reassessment Biometrics 56 1170–1176 Occurrence Handle11129475 Occurrence HandleMR1815615
H. H. Müller H. Schäfer (2001) ArticleTitleAdaptive group sequential designs for clinical trials: Combining the advantages of adaptive and of classical group sequential approaches Biometrics 57 IssueID3 886–891 Occurrence Handle11550941 Occurrence HandleMR1859823
N. Stallard (1998) ArticleTitleSample size determination for phase II clinical trials based on Bayesian decision theory Biometrics 54 279–294 Occurrence Handle9544522
M. R. Conaway G. R. Petroni (1996) ArticleTitleDesigns for phase II trials allowing for a trade-off between response and toxicity Biometrics 52 1375–1386 Occurrence Handle8962459 Occurrence HandleMR1422083
G. Verbeke G. Molenberghs H. Thijs E. Lesaffre M. G. Kenward (2001) ArticleTitleSensitivity analysis for nonrandom dropout: A local influence approach Biometrics 57 7–14 Occurrence Handle11252620 Occurrence HandleMR1833286
G. Verbeke E. Lesaffre (1999) ArticleTitleThe effect of drop-out on the efficiency of longitudinal experiments Appl. Stat 48 363–375
M. Buyse P. Thirion R. W. Carlson T. Burzykowski G. Molenberghs P. Piedbois (2000) ArticleTitleRelation between tumor response to first-line chemotherapy and survival in advanced colorectal cancer: A meta-analysis. Meta-analysis group in cancer Lancet 356 IssueID9927 373–378 Occurrence Handle10972369
M. Buyse G. Molenberghs T. Burzykowski D. Renard H. Geys (2000) ArticleTitleThe validation of surrogate endpoints in meta-analyses of randomised experiments Biostatics 1 49–67
R. Henderson P. Diggle A. Dobson (2000) ArticleTitleJoint modelling of longitudinal measurements and event time data Biostatistics 1 465–480 Occurrence Handle12933568
G. Molenberghs H. Geys M. Buyse (2001) ArticleTitleEvaluation of surrogate endpoints in randomized experiments with mixed discrete and continuous outcomes Stat. Med 20 3023–3038 Occurrence Handle11590630
S. C. D. Johnson (1998) ArticleTitleThe role of simulation in the management of research: What can the pharmaceutical industry learn from the aerospace industry? Drug inform. J 32 961–969
N. H. Holford H. C. Kimko J. P. Monteleone C. C. Peck (2000) ArticleTitleSimulation of clinical trials Ann. Rev. Pharmacol. Toxicol 40 209–234
Author information
Authors and Affiliations
Consortia
Additional information
Based on a COST B15 meeting held in Brussels 7–8 March, 2001. Members of the expert panel were: A. Boddy (U.K.), E. Chatelut (France), P. Jacqmin (Belgium), V. Levy (France), G. Paintaud (France), J. Perez-Ruixo (Spain), R. Port (Germany), D. Renard (Belgium), A. Sparreboom (USA), P. Variol (France), C. Veyrat-Follet (France)
Rights and permissions
About this article
Cite this article
Rombout, F., Aarons, L., Karlsson, M. et al. Modelling and Simulation in the Development and use of Anti-Cancer Agents: An Underused Tool?. J Pharmacokinet Pharmacodyn 31, 419–440 (2004). https://doi.org/10.1007/s10928-005-5910-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-005-5910-2