Abstract
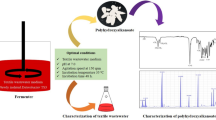
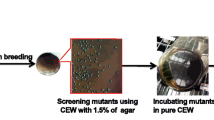
In our previous study, we found that Enterobacter strain TS3 is able to decolorize of textile wastewater (TW) through a fermentative polyhydroxyalkanoate (PHA) production process. This current study aimed to enhance the ability of strain TS3 to produce PHA using TW by a UV-light radiation-based mutagenesis system, and to enhanced PHA by multiple-round UV-light radiation. The highest PHA-production mutant was obtained in the second rounds of UV-light radiation. The radiation time was optimized to 40 s in the first round followed by 20 s at a distance of 60 cm of plates. The mutant strain TS3-UV2 yielded PHA production at 84.96 ± 1.32% cell dry mass (CDM). The highest PHA concentration (88.66 ± 1.00% CDM) is achieved at pH 7, 150 rpm and 35 °C for 60 h of incubation. Under optimal condition, the mutant yielded 0.53 times more production of PHA than the parent strain. Moreover, the decolorization efficiency of TW was observed to be 72.32% under optimal PHA conditions. Interestingly, the mutant strain could synthesize the medium-co-long-chain-length PHA (mcl-co-lcl PHA), while short-co-medium-chain-length PHA (scl-co-mcl PHA) was observed in the wild type using TW as substrate. Therefore, the mutation and optimization strategy appear to be suitable for producing high-density PHA.






Similar content being viewed by others
References
Miller R, Bates SM (2010) The majestic plastic bag-a mockumentary. https://www.plasticgarbageproject.org/en/plastic-life. Accessed 26 August 2021
Sangkharak K, Prasertsan P (2011) Utilization of biodiesel waste as a feedstock for the production of polyhydroxybutyrate by Cupriavidus necator. Afr J Biotechnol 1:17812–17824. https://doi.org/10.5897/AJB11.2184
Kemavongse K, Prasertsan P, Upaichit A, Methacanon P (2008) Poly-β-hydroxyalkanoate production by halotolerant Rhodobacter sphaeroides U7. World J Microb Bio 24:2073–2085. https://doi.org/10.1007/s11274-008-9712-8
Reddy CSK, Ghai R, Rashmi, Kalia VC (2003) Polyhydroxyalkanoates: an overview. Bioresour Technol 87:137–146. https://doi.org/10.1016/S0960-8524(02)00212-2
Sangkharak K, Prasertsan P (2007) Optimization of polyhydroxybutyrate production from wild type and two mutant strains of Rhodobacter sphaeroides using statistical method. J Biotechnol 132(3):331–340. https://doi.org/10.1016/j.jbiotec.2007.07.721
Rakkan T, Sangkharak K (2020) Enhanced decolourisation and biodegradation of textile wastewater using single and mixed cultures of a newly isolated Enterobacter strain. Curr Microbiol 77:4085–4094. https://doi.org/10.1007/s00284-020-02246-2
Kaushik P, Malik A (2009) Fungal dye decolourization: recent advances and future potential. Environ Int 35:127–141. https://doi.org/10.1016/j.envint.2008.05.010
Tamboli DP, Kagalkar AN, Jadhav MU, Jadhav JP, Govindwar SP (2010) Bioresource technology production of polyhydroxyhexadecanoic acid by using waste biomass of Sphingobacterium sp. ATM generated after degradation of textile dye Direct Red 5B. Bioresour Technol 101:2421–2427. https://doi.org/10.1016/j.biortech.2009.11.094
Joyline M, Aruna K (2019) Production and characterization of polyhydroxyalkanoates (pha) by Bacillus megaterium strain JHA using inexpensive agro-industrial wastes. Int J Recent Sci Res 10:33359–33374. https://doi.org/1010.13140/RG.2.2.36677.60641
Sangkharak K, Prasertsan P (2013) The production of polyhydroxyalkanoate by Bacillus licheniformis using sequential mutagenesis and optimization. Biotechnol Bioproc E 18:272–279. https://doi.org/10.1007/s12257-012-0615-z
Vu VH, Pham TA, Kim K (2009) Fungal strain improvement for cellulase production using repeated and sequential mutagenesis. Mycobiol 37:267–271. https://doi.org/10.4489/MYCO.2009.37.4.267
Divyashree MS, Shamala TR (2009) Effect of gamma irradiation on cell lysis and polyhydroxyalkanoate produced by Bacillus flexus. Radiat Phys Chem 78:147–152. https://doi.org/10.1016/j.radphyschem.2008.08.010
Sangkharak K, Prasertsan P (2008) Nutrient optimization for production of polyhydroxybutyrate from halotolerant photosynthetic bacteria cultivated under aerobic-dark condition. Electron J Biotechn 11:83–94. https://doi.org/10.2225/vol11-issue3-fulltext-2
Rakkan T, Chana N, Chirapongsatonkul N et al (2022) Screening and identification of Basic Red 9-degrading bacteria from textile wastewater and their ability to produce medium- and long chain length polyhydroxyalkanoate. J Polym Environ 30:415–423. https://doi.org/10.1007/s10924-021-02206-2
Hijnen WA, Beerendonk EF, Medema GJ (2006) Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo) cysts in water: a review. Water Res 40(1):3–22. https://doi.org/10.1016/j.watres.2005.10.030
Tillich UM, Lehmann S, Schulze K et al (2012) The optimal mutagen dosage to induce point mutations in Synechocystis sp. PCC6803 and its application to promote temperature tolerance. PLoS ONE 7(11):1–8. https://doi.org/10.1371/journal.pone.0049467
Black HS, deGruijl FR, Forbes PD et al (1997) Photocarcinogenesis: an overview. J Photochem Photobiol B 40(1):29–47. https://doi.org/10.1016/S1011-1344(97)00021-3
Shibai A, Takahashi Y, Ishizawa Y et al (2017) Mutation accumulation under UV radiation in Escherichia coli. Sci Rep 7:1–12. https://doi.org/10.1038/s41598-017-15008-1
Rakkan T, Chana N, Sangkharak K (2022) The integration of textile wastewater treatment with polyhydroxyalkanoate production using newly isolated Enterobacter strain TS3. Waste Biomass Valorization 13:571–582. https://doi.org/10.1007/s12649-021-01504-z
Steinbuchel A, Wiese S (1992) A Pseudomonas strain accumulating polyesters of 3-hydroxybutyric acid and medium-chain-length 3-hydroxyalkanoic acids. Appl Microbiol Biotechnol 37:691–697. https://doi.org/10.1007/BF00174829
Abe H, Doi Y, Fukushima T, Eya H (1994) Biosynthesis from gluconate of a random copolyester consisting of 3-hydroxybutyrate and medium-chain-length 3-hydroxyalkanoates by Pseudomonas sp. 61˗3. Int J Biol Macromol 16(3):115–119. https://doi.org/10.1016/0141-8130(94)90036-1
Guo W, Duan J, Geng W et al (2013) Comparison of medium-chain-length polyhydroxyalkanoates synthases from Pseudomonas mendocina NK-01 with the same substrate specificity. Microbiol Res 168(4):231–237. https://doi.org/10.1016/j.micres.2012.11.003
Steel RGD, Torrie JH (1980) Principles and procedures of statistics. McGraw-Hill, New York
Katircioglu H, Akin BS, Atici T (2004) Microalgal toxin(s): characteristics and importance. Afr J Biotechnol 3:667–674. http://www.academicjournals.org/AJB
Ashraf H, Haq IU, Qadeer MA, Iqbal J (2001) Screening of Bacillus licheniformis mutants for improved production of alpha amylase. Pak J Bot 33:518–525
Gomaa EZ (2014) Production of polyhydroxyalkanoates (PHAs) by Bacillus subtilis and Escherichia coli grown on cane molasses fortified with ethanol. Braz Arch Boil Technol 57:145–154. https://doi.org/10.1590/S1516-89132014000100020
Bhagowati P, Pradhan S, Dash HR, Das S (2015) Production, optimization and characterization of polyhydroxybutyrate, a biodegradable plastic by Bacillus spp. Biosci Biotechnol Biochem 79:1454–1463. https://doi.org/10.1080/09168451.2015.1034651
Palleroni NJ, Palleroni AV (1978) Alcaligenes latus, a new species of hydrogen utilizing bacteria. Int J Syst Bacteriol 28:41–424. https://doi.org/10.1099/00207713-28-3-416
Wei YH, Chen WC, Huang CK et al (2011) Screening and evaluation of polyhydroxybutyrate-producing strains from indigenous isolate Cupriavidus taiwanensis strains. Int J Mol Sci 12(1):252–265. https://doi.org/10.3390/ijms12010252
Arumugam A, Shereen MF (2020) Bioconversion of Calophyllum inophyllum oilcake for intensification of rhamnolipid and polyhydroxyalkanoates co-production by Enterobacter aerogenes. Bioresour Technol 296:1–6. https://doi.org/10.1016/j.biortech.2019.122321
Samrot AV, Avinesh RB, Sukeetha SD, Senthilkumar P (2011) Accumulation of poly[(R)-3-hydroxyalkanoates] in Enterobacter cloacae SU-1 during growth with two different carbon sources in batch culture. Appl Biochem Biotechnol 163:195–203. https://doi.org/10.1007/s12010-010-9028-7
Shaaban MT, Attia M, Mowafy EI (2012) Production of some biopolymers by some selective Egyptian soil bacterial isolates. J Appl Sci Res 8(1):94–105
Raj A, Kumar S, Singh SK (2013) A highly thermostable xylanase from Stenotrophomonas maltophilia: purification and characterization. Enzyme Res 2013(4):1–8. https://doi.org/10.1155/2013/429305
Javaid H, Nawaz A, Riaz N et al (2020) Biosynthesis of polyhydroxyalkanoates (PHAs) by the valorization of biomass and synthetic waste. Molecules 25(23):1–23. https://doi.org/10.3390/molecules25235539
Chen J, Zheng J, Li Y et al (2015) Characteristics of a novel thermophilic heterotrophic bacterium, Anoxybacillus contaminans HA, for nitrifcation-aerobic denitrifcation. Appl Microbiol Biotechnol 99(24):10695–10702. https://doi.org/10.1007/s00253-015-6870-0
Rakkan T, Chana N, Sangkharak K (2022) Nitrogen reduction in conjunction with polyhydroxyalkanoates production using mixed newly isolated Enterobacter. Waste Biomass Valorization 13(4):1–8. https://doi.org/10.1016/j.msea.2006.05.158
Naheed N, Jamil N (2014) Optimization of biodegradable plastic production on sugar cane molasses in Enterobacter sp. SEL2. Braz J Microbiol 45(2):417–426. https://doi.org/10.1590/S1517-83822014000200008
Singh G, Kumari A, Mittal A et al (2013) Poly β-hydroxybutyrate production by Bacillus subtilis NG220 using sugar industry waste water. Biomed Res Int 2013(4):1–10. https://doi.org/10.1155/2013/952641
Swamy J, Ramsay JA (1999) The evaluation of white rot fungi in the decoloration of textile dyes. Enzyme Microb Technol 24:130–137. https://doi.org/10.1016/S0141-0229(98)00105-7
Choonut A, Prasertsan P, Klomklao S, Sangkharak K (2020) Bacillus thermoamylovorans-related strain isolated from high temperature sites as potential producers of medium-chain-length polyhydroxyalkanoate (mcl-PHA). Curr Microbiol 77:3044–3056. https://doi.org/10.1007/s00284-020-02118-9
Kshirsagar P, Suttar R, Nilegaonkar S et al (2014) Scale up production of polyhydroxyalkanoate (PHA) at different aeration, agitation and controlled dissolved oxygen levels in fermenter using Halomonas campisalis MCM B-1027. J Biochem Technol 4(1):512–517
Zahari MAKM, Ariffin H, Mokhtar MN et al (2012) Factors affecting poly(3-hydroxybutyrate) production from oil palm frond juice by Cupriavidus necator (CCUG52238T). J Biomed Biotechnol 2012:1–8. https://doi.org/10.1155/2012/125865
Geethu M, Vrundha R, Raja S et al (2019) Improvement of the production and characterisation of polyhydroxyalkanoate by Bacillus endophyticus using inexpensive carbon feedstock. J Polym Environ 27:917–928. https://doi.org/10.1007/s10924-019-01397-z
Mohandas SP, Balan L, Lekshmi N et al (2016) Production and characterization of polyhydroxybutyrate from Vibrio harveyi MCCB 284 utilizing glycerol as carbon source. J Appl Microbiol 122(3):698–707. https://doi.org/10.1111/jam.13359
Ajar M, Skakeel S, Rehman A (2020) Microbial use for azo dye degradation-a strategy. Int Microbiol 23:149–159. https://doi.org/10.1007/s10123-019-00103-2
Poltronieri P, Kumar P (2017) Polyhydroxyalcanoates (PHAs) in industrial applications. In: Martínez L, Kharissova O, Kharisov B (eds) Handbook of Ecomaterials. Springer, Cham, pp 1–30. https://doi.org/10.1007/978-3-319-48281-1_70-1
Wecker P, Moppert X, Simon-Colin C et al (2015) Discovery of a mcl-PHA with unexpected biotechnical properties: the marine environment of French Polynesia as a source for PHA-producing bacteria. AMB Express 5(1):1–9. https://doi.org/10.1186/s13568-015-0163-y
Leyva-Porras C, Cruz-Alcantar P, Espinosa-Solís V et al (2019) Application of differential scanning calorimetry (DSC) and modulated differential scanning calorimetry (MDSC) in food and drug industries. Polymers 12(1):1–23. https://doi.org/10.3390/polym12010005
Elodie B, Patrizia C, Vera A et al (2014) Polyhydroxyalkanoate (PHA): review of synthesis, characteristics, processing and potential applications in packaging. EXPRESS Polym Lett 8:791–808. https://doi.org/10.3144/expresspolymlett.2014.82
Basnett P, Ravi S, Roy I (2016) Science and Principles of Biodegradable and Bioresorbable Medical Polymers Natural bacterial biodegradable medical polymers. pp 257–277. https://doi.org/10.1016/B978-0-08-100372-5.00008-8
Acknowledgements
The authors would like to acknowledge the support of Thailand Science Research and Innovation (TSRI) through the Royal Golden Jubilee Ph.D. (RGJ-PHD) Program through grant number PHD/00073/2559 for RGJ-PHD. Acknowledgment is also made to the Research Grant for Talented Mid-Career Researchers (SS65000399) from National Research Council of Thailand, Department of Chemistry, Faculty of Science, Thaksin University, Phatthalung Campus, Thailand and Professor Dr. Ken’ichiro MATSUMOTO.
Funding
This work was supported by Thailand Science Research and Innovation (TSRI) through the Royal Golden Jubilee Ph.D. (RGJ-PHD) Program through grant number PHD/00073/2559 for RGJ-PHD; The Research Grant for Talented Mid-Career Researchers (N42A650241) from National Research Council of Thailand (NRCT); Thaksin University.
Author information
Authors and Affiliations
Contributions
TR: methodology, writing-original draft, data collection and analysis. NC: mentoring. KS: conceptualization, writing-review and editing, visualization, supervision, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rakkan, T., Chana, N. & Sangkharak, K. Utilization of Textile Wastewater as A Substrate for Polyhydroxyalkanoate (PHA) and Enhanced Production by Mutant Enterobacter. J Polym Environ 31, 677–687 (2023). https://doi.org/10.1007/s10924-022-02563-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-022-02563-6