Abstract
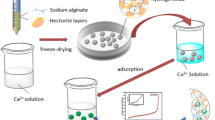
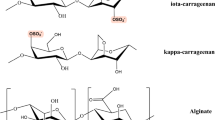
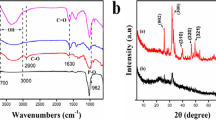
Based on the excellent performances of nanocrystalline cellulose, sodium alginate, or K-carrageenan in Pb2+ adsorption, nanocrystalline cellulose/sodium alginate/K-carrageenan composite hydrogel beads were prepared to adsorb Pb(II) from aqueous solutions. The objective of this study was to demonstrate the excellent potential of the composite hydrogel beads for heavy metal ion adsorption. We successfully prepared ecofriendly Fe-modified nanocrystalline cellulose/sodium alginate/K-carrageenan composite hydrogel beads and characterized them. The structure and adsorption mechanism were investigated using scanning electron microscopy, Fourier-transform infrared spectroscopy, and X-ray photoelectron spectroscopy, and the optimal adsorption conditions were determined. The tricomponent hydrogel beads were robust and exhibited improved adsorption capacity for Pb2+ and good reusability. The adsorption results could be fitted well with a pseudo-second-order kinetic model and the Langmuir adsorption model. The maximum adsorption capacity obtained by fitting was 351.04 mg g−1. Recycling experiments revealed that the adsorption capacity of the adsorbent remained high after five cycles of reuse.










Similar content being viewed by others
Abbreviations
- CNC:
-
Nanocrystalline cellulose
- FTIR:
-
Fourier-transform infrared
- SEM:
-
Scanning electron microscopy
- EDX:
-
Energy-dispersive X-ray spectroscopy
- XPS:
-
X-ray photoelectron spectroscopy
- PFO:
-
Pseudo first order
- PSO:
-
Pseudo second order
References
Fry KL, Wheeler CA, Gillings MM, Russell Flegal A, Taylor MP (2020) Anthropogenic contamination of residential environments from smelter As, Cu and Pb emissions: implications for human health. Environ Pollut 262:114235
Dragan ES, Cocarta AI, Dinu MV (2014) Facile fabrication of chitosan/poly(vinyl amine) composite beads with enhanced sorption of Cu2+. Equilibrium, kinetics, and thermodynamics. Chem Eng J 255:659–669
Liang XX, Ouyang X-K, Wang S, Yang L-Y, Huang F, Ji C, Chen X (2019) Efficient adsorption of Pb(II) from aqueous solutions using aminopropyltriethoxysilane-modified magnetic attapulgite@chitosan (APTS-Fe3O4/APT@CS) composite hydrogel beads. Int J Biol Macromol 137:741–750
Chen Y, Long Y, Li Q, Chen X, Xi Xu (2019) Synthesis of high-performance sodium carboxymethyl cellulose-based adsorbent for effective removal of methylene blue and Pb (II). Int J Biol Macromol 126:107–117
Dhaouadi F, Sellaoui L, Badawi M, Reynel-Ávila HE, Mendoza-Castillo DI, Jaime-Leal JE, Bonilla-Petriciolet A, Lamine AB (2020) Statistical physics interpretation of the adsorption mechanism of Pb2+, Cd2+ and Ni2+ on chicken feathers. J Mol Liquids 319:114168
Liu Y, Gao Q, Li C, Liu S, Xia K, Han B, Zhou C (2019) Effective coating of crosslinked polyethyleneimine on elastic spongy monolith for highly efficient batch and continuous flow adsorption of Pb(II) and acidic red 18. Chem Eng J. https://doi.org/10.1016/j.cej.2019.123610
Keshav V, Achilonu I, Dirr HW, Kondiah K (2019) Recombinant expression and purification of a functional bacterial metallo-chaperone PbrD-fusion construct as a potential biosorbent for Pb(II). Protein Expr Purif 158:27–35
Sahu S, Pahi S, Tripathy S, Singh SK, Behera A, Sahu UK, Patel RK (2020) Adsorption of methylene blue on chemically modified lychee seed biochar: dynamic, equilibrium, and thermodynamic study. J Mol Liquids 315:113743
Bozbas SK, Ay U, Kayan A (2013) Novel inorganic-organic hybrid polymers to remove heavy metals from aqueous solution. Desalin Water Treat 51:7208–7215
Kayan A (2019) Inorganic-organic hybrid materials and their adsorbent properties. Adv Compos Hybrid Mater 2:34–45
Kayan GO, Kayan A (2021) Composite of natural polymers and their adsorbent properties on the dyes and heavy metal ions. J Polym Environ. https://doi.org/10.1007/s10924-021-02154-x
Li C, Wang XJ, Meng DY, Zhou L (2018) Facile synthesis of low-cost magnetic biosorbent from peach gum polysaccharide for selective and efficient removal of cationic dyes. Int J Biol Macromol 107:1871–1878
Ebrahimzadeh H, Asgharinezhad AA, Moazzen E, Amini MM, Sadeghi O (2015) A magnetic ion-imprinted polymer for lead(II) determination: a study on the adsorption of lead(II) by beverages. J Food Compos Anal 41:74–80
Chamchoy K, Inprasit T, Vanichvattanadecha C, Thiangtrong A, Anukunwithaya P, Pisitsak P (2021) The magnetic properties and dye adsorption of sericin-modified magnetite nanoparticles. J Polym Environ 29:484–491
Song Y, Yang L-Y, Wang Y-G, Di Yu, Shen J, Ouyang X-K (2019) Highly efficient adsorption of Pb(II) from aqueous solution using amino-functionalized SBA-15/calcium alginate microspheres as adsorbent. Int J Biol Macromol 125:808–819
Agnihotri S, Singhal R (2019) Effect of sodium alginate content in acrylic acid/sodium humate/sodium alginate superabsorbent hydrogel on removal capacity of MB and CV dye by adsorption. J Polym Environ 27:372–385
Li R, Liu Y, Lan G, Qiu H, Xu B, Xu Q, Sun N, Zhang L (2021) Pb(II) adsorption characteristics of magnetic GO-hydroxyapatite and the contribution of GO to enhance its acid resistance. Journal of Environmental Chemical Engineering 9:105310
Liu C, Omer AM, Ouyang X-k (2018) Adsorptive removal of cationic methylene blue dye using carboxymethyl cellulose/k-carrageenan/activated montmorillonite composite beads: isotherm and kinetic studies. Int J Biol Macromol 106:823–833
Wang F, Duo TT, Wang YX, Xiao ZH, Xu AR, Liu RK (2021) Novel polyethyleneimine/kappa-carrageenan composite from facile one-step fabrication for the removal of copper ion from aqueous solution. J Polym Environ. https://doi.org/10.1007/s10924-021-02247-7
Qu J, Tian X, Jiang Z, Cao B, Akindolie MS, Hu Q, Feng C, Feng Y, Meng X, Zhang Y (2020) Multi-component adsorption of Pb(II), Cd(II) and Ni(II) onto microwave-functionalized cellulose: kinetics, isotherms, thermodynamics, mechanisms and application for electroplating wastewater purification. J Hazard Mater 387:121718
Araga R, Sharma CS (2019) Amine functionalized electrospun cellulose nanofibers for fluoride adsorption from drinking water. J Polym Environ 27:816–826
Montes L, Gisbert M, Hinojosa I, Sineiro J, Moreira R (2021) Impact of drying on the sodium alginate obtained after polyphenols ultrasound-assisted extraction from Ascophyllum nodosum seaweeds. Carbohydrate Polymers 272:118455
Fan L, Yuqing Lu, Yang L-Y, Huang F, Ouyang X-K (2019) Fabrication of polyethylenimine-functionalized sodium alginate/cellulose nanocrystal/polyvinyl alcohol core–shell microspheres ((PVA/SA/CNC)@PEI) for diclofenac sodium adsorption. J Colloid Interface Sci 554:48–58
Li L, Zhao J, Sun Y, Fei Yu, Ma J (2019) Ionically cross-linked sodium alginate/ĸ-carrageenan double-network gel beads with low-swelling, enhanced mechanical properties, and excellent adsorption performance. Chem Eng J 372:1091–1103
Yu F, Cui T, Yang C, Dai X, Ma J (2019) κ-Carrageenan/Sodium alginate double-network hydrogel with enhanced mechanical properties, anti-swelling, and adsorption capacity. Chemosphere 237:124417
Najafpoor A, Norouzian-Ostad R, Alidadi H, Rohani-Bastami T, Davoudi M, Barjasteh-Askari F, Zanganeh J (2020) Effect of magnetic nanoparticles and silver-loaded magnetic nanoparticles on advanced wastewater treatment and disinfection. J Mol Liquids 303:112640
Alsuhybani M, Alshahrani A, Algamdi M, Al-Kahtani AA, Alqadami AA (2020) Highly efficient removal of Pb(II) from aqueous systems using a new nanocomposite: adsorption, isotherm, kinetic and mechanism studies. J Mol Liquids 301:112393
Huang B, Lu MC, Wang DL, Song YH, Zhou L (2018) Versatile magnetic gel from peach gum polysaccharide for efficient adsorption of Pb2+ and Cd2+ ions and catalysis. Carbohyd Polym 181:785–792
Tan KB, Reza AK, Abdullah AZ, Horri BA, Salamatinia B (2018) Development of self-assembled nanocrystalline cellulose as a promising practical adsorbent for methylene blue removal. Carbohydr Polym 199:92–101
Qinghua Xu, Wang Y, Liqiang Jin Yu, Wang MQ (2017) Adsorption of Cu (II), Pb (II) and Cr (VI) from aqueous solutions using black wattle tannin-immobilized nanocellulose. J Hazard Mater 339:91–99
Hashem A, Fletcher AJ, Younis H, Mauof H, Abou-Okeil A (2020) Adsorption of Pb(II) ions from contaminated water by 1,2,3,4-butanetetracarboxylic acid-modified microcrystalline cellulose: isotherms, kinetics, and thermodynamic studies. Int J Biol Macromol 164:3193–3203
Wang N, Ouyang X-K, Yang L-Y, Omer AM (2017) Fabrication of a magnetic cellulose nanocrystal/metal–organic framework composite for removal of Pb(II) from water. ACS Sustain Chem Eng 5:10447–10458
Jiang F, Zhang D, Ouyang X-k, Yang L-Y (2021) Fabrication of porous polyethyleneimine-functionalized chitosan/Span 80 microspheres for adsorption of diclofenac sodium from aqueous solutions. Sustain Chem Pharm 21:100418
Abdi G, Alizadeh A, Amirian J, Rezaei S, Sharma G (2019) Polyamine-modified magnetic graphene oxide surface: feasible adsorbent for removal of dyes. J Mol Liquids 289:111118
Alqadami AA, Naushad M, Abdalla MA, Ahamad T, Alothman ZA, Alshehri SM, Ghfar AA (2017) Efficient removal of toxic metal ions from wastewater using a recyclable nanocomposite: a study of adsorption parameters and interaction mechanism. J Clean Prod 156:426–436
Zhang Y, Xia M, Wang F, Ma J (2021) Experimental and theoretical study on the adsorption mechanism of Amino trimethylphosphate (ATMP) functionalized hydroxyapatite on Pb (II) and Cd (II). Colloids Surf A Physicochem Eng Asp 626:12729
Ahmad R, Mirza A (2015) Sequestration of heavy metal ions by Methionine modified bentonite/Alginate (Meth-bent/Alg): a bionanocomposite. Groundw Sustain Dev 1:50–58
Kulal P, Badalamoole V (2020) Hybrid nanocomposite of kappa-carrageenan and magnetite as adsorbent material for water purification. Int J Biol Macromol 165:542–553
Zhang J, Li T, Li X, Liu Y, Li N, Wang Y, Li X (2021) A key role of inner-cation-pi interaction in adsorption of Pb(II) on carbon nanotubes: experimental and DFT studies. J Hazard Mater 412:125187
Wang C, Xiong C, He Y, Yang C, Li X, Zheng J, Wang S (2021) Facile preparation of magnetic Zr-MOF for adsorption of Pb(II) and Cr(VI) from water: adsorption characteristics and mechanisms. Chem Eng J 415:128923
Hu ZH, Omer AM, Ouyang XK, Yu D (2018) Fabrication of carboxylated cellulose nanocrystal/sodium alginate hydrogel beads for adsorption of Pb(II) from aqueous solution. Int J Biol Macromol 108:149–157
Jafarnejad M, Asli MD, Taromi FA, Manoochehri M (2020) Synthesis of multi-functionalized Fe3O4–NH2-SH nanofiber based on chitosan for single and simultaneous adsorption of Pb(II) and Ni(II) from aqueous system. Int J Biol Macromol 148:201–217
Lian Q, Ahmad ZU, Gang DD, Zappi ME, Fortela DLB, Hernandez R (2020) The effects of carbon disulfide driven functionalization on graphene oxide for enhanced Pb(II) adsorption: Investigation of adsorption mechanism. Chemosphere 248:126078
Jiang Q, Xie W, Han S, Wang Y, Zhang Y (2019) Enhanced adsorption of Pb(II) onto modified hydrochar by polyethyleneimine or H3PO4: an analysis of surface property and interface mechanism. Colloids Surf A Physicochem Eng Asp 583:123962
Ahmed W, Mehmood S, Nunez-Delgado A, Ali S, Qaswar M, Shakoor A, Mahmood M, Chen DY (2021) Enhanced adsorption of aqueous Pb(II) by modified biochar produced through pyrolysis of watermelon seeds. Sci Total Environ 784:147136
Chen Y, Wang S, Li Y, Liu Y, Chen Y, Wu Y, Zhang J, Li H, Peng Z, Xu R, Zeng Z (2020) Adsorption of Pb(II) by tourmaline-montmorillonite composite in aqueous phase. J Colloid Interface Sci 575:367–376
van Veenhuyzen B, Tichapondwa S, Hörstmann C, Chirwa E, Brink HG (2021) High capacity Pb(II) adsorption characteristics onto raw- and chemically activated waste activated sludge. J Hazard Mater 416:125943
Hashem A, Aniagor CO, Taha GM, Fikry M (2021) Utilization of low-cost sugarcane waste for the adsorption of aqueous Pb(II): kinetics and isotherm studies. Curr Res Green Sustain Chem 4:100056
Miao Y, Peng W, Cao Y, Chang L, Fan G, Yu F (2021) Facile preparation of sulfhydryl modified montmorillonite nanosheets hydrogel and its enhancement for Pb(II) adsorption. Chemosphere 280:130727
Wang Z, Xu J, Yellezuome D, Liu R (2021) Effects of cotton straw-derived biochar under different pyrolysis conditions on Pb (II) adsorption properties in aqueous solutions. J Anal Appl Pyrolysis 157:105214
Han X, Li P, Zhang M, Wang J, Cao Y, Zhang T, Zhou G, Li F (2021) Designing three-dimensional half-embedded ES-PAN/AHCNs adsorption membrane for removal of Pb(II), Cu(II) and Cr(III). Colloids Surf A Physicochem Eng Asp 627:127177
Shi S, Dong Q, Wang Y, Zhang X, Zhu S, Chow YT, Wang X, Zhu L, Zhang G, Xu D (2021) Self-supporting super hydrophilic MgFe2O4 flexible fibers for Pb(II) adsorption. Sep Purif Technol 266:118584
Xu X, Ouyang X-k, Yang L-Y (2021) Adsorption of Pb(II) from aqueous solutions using crosslinked carboxylated chitosan/carboxylated nanocellulose hydrogel beads. J Mol Liquids 322:114523
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 21606136) and Zhejiang Provincial Key Laboratory of Health Risk Appraisal for Trace Toxic Chemicals (Grant Nos. 2021001, 2021002).
Author information
Authors and Affiliations
Contributions
JS: Investigation, Funding acquisition. FJ: Investigation, Data curation, writing-Original Draft. X-kO: Conceptualization, Methodology, Funding acquisition. M-cJ: Supervision, Funding acquisition.
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shen, J., Xu, X., Ouyang, Xk. et al. Adsorption of Pb(II) from Aqueous Solutions Using Nanocrystalline Cellulose/Sodium Alginate/K-Carrageenan Composite Hydrogel Beads. J Polym Environ 30, 1995–2006 (2022). https://doi.org/10.1007/s10924-021-02334-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-021-02334-9