Abstract
This study was focused on the synthesis and characterization of a fully bio-based unsaturated polyester resin (UPR) with good thermal properties suitable for the commercial production of composite polymers. UPRs based on different ratios of bio-based furan dicarboxylic acid (FDCA), itaconic acid, and diols were synthesized. The unsaturated polymers prepared were evaluated by differential scanning calorimetry, gel permeation chromatography, FTIR and 1H-NMR spectroscopy. The results showed positive effects of FDCA on the glass transition temperature (Tg) of these fully bio-based polyesters, especially when FDCA was combined with 1,2-propanediol. Optimal values of Tg were obtained in the range of 30–32 °C for UPRs synthesized starting with a higher concentration of FDCA in the monomer feed. The possibility of substituting styrene, which usually acts as a reactive diluent, with a greener and safer alternative during the crosslinking of UPRs, was also explored. Two bio-based reactive diluents were considered: dimethyl itaconate (DMI) and butanediol dimethacrylate. After crosslinking, an average Tg of 75 °C and a good crosslinking efficiency indicated by a gel content of 90% were achieved for the fully bio-based polyester obtained under milder reaction conditions and dilution with DMI. Life cycle assessment was performed on selected UPRs, and comparison with a reference fossil-based resin in terms of the calculated category indicator results confirmed the lower environmental impact of the newly prepared bio-based polyesters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Unsaturated polyesters (UPEs) are among the most versatile synthetic resins used in the manufacture of composite polymers [1, 2]. Unsaturated polyester resins (UPRs) have a wide range of applications, for example, in the construction sector, as well as in the field of transportation, tissue engineering and electronics [3,4,5]. Important applications of UPEs can be found in automotive, electric and electronic sectors as well as in military products, furniture, sport, leisure, domestic and sanitary appliances [6, 7]. UPEs have recently been used in bridge construction and general infrastructure repairs, as well as for railways, wagon system manufacturing, and in some naval structural elements such as bulkheads, decks, masts and propellers [8]. The two main advantages of UPEs are their very good structural properties and their low cost [9]. For these reasons, they are considered high-value, low-cost resins with a high performance/cost ratio [10].Other advantages include easy handling and a good combination of mechanical, electrical and chemical properties [9].
The incorporation of low molecular weight monomers as reactive diluents, e.g. styrene, which is the most widely used [11], enables the final properties of the cured UPR to be tuned, for example, the viscosity and processability, and the thermal and mechanical properties [12]. However, styrene is associated with several health and safety problems due to its flammability, volatility and hazardous character. Moreover, the development of styrene-free bio-based resins is considered an effective way to avoid the use of fossil-based resources and to produce high-performance materials [13,14,15].
We are currently in a transition from a fossil-based economy to the more sustainable production of industrial chemicals and materials compliant with a circular economy. This has led to interest among researchers and scientists in the integration of renewable resources as sustainable raw materials in the manufacture of UPRs [16,17,18]. A variety of bio-based thermoplastic polyesters, such as poly(lactic acid), polyhydroxyalkanoates and poly(butylene succinate), have been developed and successfully commercialized [19]. However, only a small number of studies have been carried out on bio-based thermosetting UPRs and few commercial products are available, despite their attractive properties and wide range of applications [20].
Difficulties in producing high-performance, low-cost UPRs from bio-based raw materials have led to only the partial replacement of fossil-based materials with bio-based counterparts. For instance, an orthophthalic UPR has been substituted with up to 25 wt% bio-based epoxidized methyl linseedate, and a decrease in storage modulus, crosslinking density, and glass transition temperature was reported with increasing amount of the bio-based modifier [10]. The use of 2,5-furan dicarboxylic acid (2,5-FDCA) as a substitute for petroleum-derived terephthalic acid (TPA) to obtain bio-based polyesters via condensation polymerization with different diols has been demonstrated in several studies [21,22,23]. In recent years, the successful use of itaconic acid (IA) as an unsaturated building block for the synthesis of renewable polyesters has been demonstrated [24, 25].
The synthesis of a range of fully bio-based UPEs, via melt polycondensation of IA, 2,5-FDCA, succinic acid, and 1,3-propanediol, has recently been reported [26]. The effect of using dialkyl itaconates as reactive diluents in a series of 100% bio-based UPRs prepared from IA and 1,2-propanediol (1,2-PD) has also be investigated [27]. The development of a 100% renewable polyester binder from bio-based monomers, and its environmental impact, have been presented in our previous study [28]. However, to the best of our knowledge, no studies have yet been carried out on the synthesis and characterization of fully bio-based thermosetting UPRs based on 2,5-FDCA and IA, including evaluations of the effects of bio-based UPRs and green reactive diluents on greenhouse gas (GHG) emissions and the total non-renewable energy use (NREU).
In the light of the above, this study was undertaken in an attempt to develop a fully bio-based UPR, in terms of both polyester synthesis and formulation with alternative reactive diluents. The aim was to further our knowledge on the sustainable production of industrial chemicals, and to reduce the environmental impact of thermoset composite materials. Fully bio-based polyesters were obtained via polycondensation between bioderived diols and dicarboxylic acids. Among the bioderived dicarboxylic acid monomers, 2,5-FDCA was chosen as a green alternative to the commonly used fossil-derived TPA, which has a similar chemical structure to FDCA. This bio-based dicarboxylic acid can be obtained from polysaccharides, starch, or lignocellulosic biomass [29]. Thanks to the presence of the heterocyclic ring in 2,5-FDCA, the synthesis of a polyester with a high Tg and a high degree of stiffness can be produced, improving the mechanical properties of the final resin [26], as well as its chemical resistance. IA is a carbohydrate-derived aliphatic dicarboxylic acid, and was chosen as a bioderived alternative to the commonly used fossil-based maleic anhydride. Its structure is characterized by the presence of two carboxyl groups and one carbon–carbon double bond. It provides the unsaturation sites in the final polyester structure, which are fundamental to the initiation of the crosslinking process in thermosetting unsaturated resins [27]. 1,2-PD and 1,3-propandiol (1,3-PD) were used as green alternatives to commonly used fossil-based diols. The main objectives of this study were: (i) to synthesize and characterize a fully bio-based thermosetting UPR, (ii) to substitute the commonly used styrene with a greener and safer reactive diluent for resin preparation, and (iii) to perform life cycle assessment (LCA) for selected UPR formulations. Two environmental impact categories were compared with those of a fossil-based resin, clearly showing the benefit of using a 100% bio-based reactive diluent.
Experimental Section
Materials
The two acid monomers used for polycondensation were 2,5-FDCA (purity 99%) and IA (99%). 1,2-PD and 1,3-PD, both with a purity of 98%, were used as diols. All the monomers were purchased from Sigma-Aldrich S.r.l. (Milan, Italy) and used as received. During the reactions, catalysts, antioxidants, and inhibitors of free radical polymerization were also added. Stannous octoate [Sn(Oct)2, purity 92.5%] and titanium (IV) isopropoxide (TTIP, purity 97%) were used as catalysts (also purchased from Sigma-Aldrich S.r.l.). A liquid antioxidant phosphite, WESTON 705 (P% = 5%), was supplied by Addivant, while toluhydroquinone (purity ≥ 99%, THQ) was purchased from Sigma-Aldrich S.r.l., and used as an inhibitor. Dimethyl itaconate (DMI) or 1,4-butanediol dimethyl methacrylate (BDM) were used as reactive diluents as alternatives to styrene. Benzoyl peroxide (BPO, 75%) and methyl ethyl ketone peroxide (MEKP, 75%) were used to initiate crosslinking. Cobalt (II) naphthenate (CoNAP) containing 6% of Co was used as a catalyst to accelerate crosslinking. Potassium hydroxide solution in ethanol (KOH, 0.1 mol/L) and phenolphthalein titration solution in tetrahydrofuran (THF), were used at a ratio of phenolphthalein/THF = 0.5 mg/100 mL, to determine the acid value (AV) in a dissolved resin sample. THF was also used as a diluent for the gel content calculation. All the materials used for the evaluation of the AV, the crosslinking procedure, gel content determination and the dilution of polyesters were kindly provided by Sigma-Aldrich S.r.l.
Synthesis of Unsaturated Polyester Resins
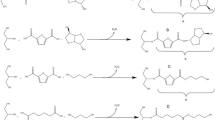
UPRs were obtained via polycondensation of two different bifunctional acid monomers (2,5-FDCA and IA) and either one diol or two diols, according to the synthesis reaction. Synthesis was divided into two steps: pre-polymerization between FDCA and the diols, followed by the addition of the unsaturated IA to complete the polycondensation reaction.
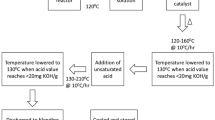
In the first step, either 1,3-PD or 1,2-PD, or a mixture of them, and 2,5-FDCA were directly introduced into the reaction flask, together with p-toluene sulfuric acid monohydrate (0.16–0.18 wt% based on the total weight of all monomers). The reaction was carried out in a closed Dean-Stark system with a stirrer, and a thermometer, under a nitrogen atmosphere. The temperature in the flask was gradually increased from room temperature to 120 °C at a rate of approximately 1 °C/min, and then kept constant. The heating rate was slow enough to avoid reagent loss due to evaporation. Either 0.1 wt% of Sn(Oct)2 or 0.4 wt% of TTIP (percentages based on the total weight of all monomers) was added only when the increasing temperature reached the values range from 115 to 140 °C. Pre-polymerization was allowed to progress until complete dissolution of the solid 2,5-FDCA in the diols was achieved, i.e. until the mixture became transparent. The reaction was then interrupted by cooling the mixture to room temperature, while THQ was added as a radical inhibitor (0.01 wt% based on the total weight of all monomers).
In the second step, the mixture was again heated while adding IA. The antioxidant liquid phosphite WESTON 75 (0.06–0.08 wt% based on the initial total weight of all monomers) was added to the reaction flask, together with 0.1 wt% of Sn(Oct)2 or 0.4 wt% of TTIP with respect to the total weight of all monomers. The temperature of the mixture was increased slowly until evidence of the progress of the reaction was seen, i.e. the temperature of the distillation head increased and water condensed as a by-product, which was continuously removed from the mixture. When the temperature of the distillation head reached 60–70 °C (indicating an adequate reaction rate), the temperature of the mixture was kept constant. When the temperature of the distillation head started to decrease, the temperature of the mixture was further increased to facilitate the progress of reaction, up to a maximum temperature of approximately 160 °C when TTIP was used as the catalyst and 200 °C when Sn(Oct)2 was used, and then kept constant. Thereafter, the progress of the reaction was monitored by determining the residual AV, until a value between 15 and 20 mg KOH/g was obtained. The properties of the final polymer were investigated using FTIR, and 1H-NMR spectroscopy, gel permeation chromatography (GPC) and differential scanning calorimetry (DSC).
Resin Crosslinking
To perform crosslinking, the polyester was dissolved in each diluent, either DMI or BDM, at a polymer/diluent weight ratio of 70/30. 1 wt% BPO, 1.5 wt% MEKP and 0.4 wt% CoNAP, were added to obtain a crosslinked material. The crosslinking process consisted of an initial step at room temperature for 24 h, followed by heating for 3 h at 80 °C, a thermal ramp from 80 to 160 °C over 0.5 h, and final heating for 3 h at 160 °C.
Characterization Methods
1H-NMR (400 MHz) spectra were recorded on a Bruker Avance 400. Chemical shifts were expressed as δ values downfield from tetramethylsilane (TMS), and dimethyl sulfoxide-d6 was used as the diluent for the polyesters under investigation. The FTIR spectra were recorded with a Thermo Nicolet Nexus 670 FTIR spectrometer. For FTIR analysis, polyester samples were dissolved in THF, deposited on a transparent KBr window and analyzed after diluent evaporation. Each spectrum was acquired by averaging over 64 scans, with a resolution of 4 cm−1 in the region 400 to 4000 cm−1. A Waters 515 HPLC system was used for GPC studies (using THF as the mobile phase, at a flow rate of 1 mL/min at 35 °C), equipped with a system of columns connected in series (Ultrastyragel HR, Waters), and a refractive index detector (Waters 2410). Samples were dissolved in THF at a concentration of 0.2 wt%. The GPC system was calibrated with monodispersed fractions of linear polystyrene standards in the molecular weight range 102–106 g/mol. DSC analysis was performed with a DSC 823e calorimeter (Mettler-Toledo). Both pure polyesters and crosslinked resins were tested with the following thermal cycle: (i) a heating ramp from − 50 to 200 °C,(ii) a cooling run from 200 to − 50 °C, and (iii) a further heating run from − 50 to 200 °C, with heating and cooling rates of 20 °C/min under an inert nitrogen atmosphere. The glass transition temperature (Tg) was determined as the inflection point of the curve. Gel content (G%) was determined as a measure of the degree of crosslinking of the polyester resins. The solid specimens were placed in THF at room temperature (the THF/\({m}_{0}\) ratio was 0.15 mL/mg) and stirred with magnetic stirring for 24 h, to extract not crosslinked polymer chains from the solid polymer network. The gel content was determined according to the following equation:
where \({m}_{0}\) is the initial mass of the sample and \({m}_{e}\) is the mass of not crosslinked materials extracted.
Life Cycle Assessment
The methodological framework of LCA given in the ISO standards 14040/44 [30, 31] was used for LCA in this study.
Goal and Scope of the LCA
LCA of the unsaturated polyesters made from renewable resources was performed to determine the environmental impact associated with their production, and the results were compared to those for a fossil-based resin. All the monomers, the polymerization process and diluents used in the production of the unsaturated polyesters were considered.
Impact Categories Considered in the LCA
Only greenhouse gas (GHG) emissions and the total non-renewable energy use (NREU) were considered in the LCA. Although other categories, such as water consumption, land use and eutrophication, are also important when assessing the effects of biomass-derived products, the data used were taken from different sources, and homogeneous data could only be found for GHG and NREU. The characterization factors required for the calculations were taken from the methods estimating the impact presented in these documents: “Greenhouse Gas Protocol” (v1.02) and “Cumulative Energy Demand” (v1.10). The latter is based on the 100-year time frame global warming potential (GWP), and the 100-year IPCC GWPs were thus used to calculate carbon dioxide equivalents (CO2eq) of all non-CO2 gases (CH4, N2O, SF6, HFCs and CFCs) [32].
Functional Unit and System Boundaries
Figure 1 shows the system boundaries for the different UPEs analyzed. The functional unit is one kg of unsaturated polyester, and a fossil-based unsaturated polyester (UPE1) was compared with a partially bio-based unsaturated polyester (UPE2) and a fully bio-based unsaturated polyester (UPE3). The system boundary was defined following a cradle-to-factory-gate approach, including resource extraction, transportation to the monomer factory, production of raw monomers and polyester production (before being transported to the end-user), as shown in Fig. 1. The contributions of the different monomers, the polymerization process, and the different diluents were analyzed separately up to the step of polyester production. As can be seen from Fig. 1, the only difference between UPE2 and UPE3 lies in the diluent. The diluent 2 (butanediol dimethacrylate) is partially bio-based and therefore non-renewable feedstock was used for diluent 2, while the diluent 3 (dimethyl itaconate) is fully bio-based.
The system boundaries of the three different unsaturated polyesters. a UPE1: fossil-based, b UPE2: partially bio-based, and c UPE3: 100% bio-based. Orange boxes indicate monomers from fossil-based sources (IAUPE and MetA indicate an isophthalic acid-based unsaturated polyester and methacrylic acid, respectively). Green boxes indicate monomers based on renewable resources
Corn-based itaconic acid (IA_C), biodiesel glycerol-based bio-based 1,2-PD (1,2-PD_BDG) and wheat straw-based bio-based 1,4-butanediol (1,4-BD_WS) were chosen as the base cases in this study because their features were closer to the materials used for the polymerizations (further details are given in Table 1).
Life Cycle Inventory Analysis
Most of the data (secondary data) were obtained from the literature (Table 1). These data were then incorporated into SimaPro LCA software, and the Ecoinvent database (version 3.5), an attributional system model, was used as a background source. The foreground data were then evaluated. The inputs and outputs considered in the life cycle inventory are given in Table 2.
Sensitivity Analysis
To determine how the LCA is affected by the input parameters, a sensitivity analysis was performed using information available in the literature on the production of itaconic acid, 1,2-PD and 1,4-butanediol in the specific cases of UPE2 and UPE3 from different biomasses. In the case of 2,5-FDCA, no such analysis was possible due to a lack of information in the literature. The sensitivity analysis was carried out on the basis of ISO standard [31].
Itaconic acid derived from corn (IA_C) was replaced by itaconic acid derived from wood (IA_W) to investigate the results on both GHG emissions and NREU. Bio-based 1,2-PD derived from biodiesel glycerol (1,2-PD_BDG) was replaced by bio-based 1,2-PD originating from algae (1,2-PD_Alg). In this case, only the effects on GHG emissions could be analyzed due to a lack of information on NREU in the literature. Bio-based 1,4-butanediol from wheat straw (1,4-BD_WS) was replaced with bio-based 1,4-butanediol derived from corn stover (1,4-BD_CS). Even in this case, only the effects on GHG emissions could be analyzed. More details are shown in Table 1.
Results and Discussion
Unsaturated Polyester Synthesis and Characterization
The main aim of this study was to produce an unsaturated polyester from 100% renewable carbon sources, with properties suitable for the manufacture of composite polymers, such as good crosslinking (as close as possible to 100% gel content), and a polymer Tg of at least + 60 °C to afford a high level of stiffness for outdoor applications. Fully bio-based polyesters were obtained in this work via polycondensation between bioderived diols, i.e. 1,2-PD and 1,3-PD and two dicarboxylic acids, i.e. 2,5-FDCA and IA.
However, some problems were encountered during the polycondensation, due to the physical properties and poor reactivity of 2,5-FDCA. 2,5-FDCA is a solid with a melting point at about 342 °C, which is higher than that of IA. Moreover, 2,5-FDCA has a lower solubility than IA in most organic diluents, including alcohols and acetates [39, 40]. In addition, it exhibits lower reactivity due to its bulkier structure, and the presence of two secondary acid groups. As a result of 2,5-FDCA properties and reactivity, the formation of heterogeneous phases was observed when a single-step polycondensation reaction was carried out, due to the ineffective randomization of the monomers in the polymer backbone. To overcome this problem, a two-step polycondensation was performed. The first stage, pre-polymerization, involved the reaction of 2,5-FDCA with the diols until complete homogeneity was observed, followed by a second stage, starting with the addition of IA. Moreover, problems related to the cyclization of the diols were encountered when 1,4-butanediol was used as a diol in our previous experiments. This was addressed and avoided using 1,2-PD and 1,3,-PD. As 1,2-PD and 1,3,-PD are among the shortest of the bio-based diols currently available, their use enabled the formation of a rigid polyester backbone. Furthermore, the bulkier structure of 1,2-PD helped to increase the stiffness of the polyester.
Various ratios of the monomers described above were used to synthesize different unsaturated polyesters, as summarized in Table 3, and to obtain an optimal structure with a high Tg, in view of potential applications in composite manufacturing. During our experimental study, it was observed that increasing the amount of 2,5-FDCA could lead to a higher value of Tg in the polymer. Thus, the amount of 2,5-FDCA was gradually increased, to achieve as high Tg values as possible, while ensuring that the reaction conditions were sufficiently mild to allow the introduction of the heat-sensitive IA monomer. Furthermore, during the polycondensation, attention was paid to the possibility of premature crosslinking of the mixture, due to the presence of polymer segments with a high density of unsaturation, which would have led to increased reactivity of that segment.
As the amount of FDCA was increased, the amount of IA had to be reduced to maintain the molar ratio of OH/COOH (i.e. 1.2). The total molar percentage of diols was kept constant at 54.8% mol while investigating the effects of the different diols. The progress of the polycondensation reaction was followed by determining the AV at different times, as AV is normally proportional to the degree of polymerization. Two different types of catalysts were employed, i.e. Sn(Oct)2 and TTIP. In the latter case, milder conditions, i.e. with a maximum temperature of 160 °C, could be employed to synthetize UP05, which showed values of AV, Mn and PDI comparable to those measured for UP04 obtained with Sn(Oct)2 and same concentrations of monomers.
Among the synthetized polyesters reported in Table 3, UP04 and UP05 were chosen as suitable polyesters for further investigation as their higher values of Tg (30 and 32 °C, respectively) indicated that they would be suitable for the preparation of a stiffer, heat-resistant polymer. The structure of these unsaturated polyesters was investigated by FTIR and 1H-NMR spectroscopy. Figure 2 shows the FTIR spectra of the two polyesters, both of which showed an absorption peak at 3560 cm−1 attributable to the –OH group. The peaks around 3000–2850 cm−1 were associated with –CH– stretching, confirming the presence of aliphatic groups. The strong absorption peak at 1750 cm−1 was attributed to the carbonyl group –C=O of the ester group, while the peaks between 1500 and 1700 cm−1 were assigned to the unsaturation sites of FDCA and IA at 1582 cm−1 and 1642 cm−1, respectively. According to the literature, the other peaks at 1120 cm−1 and 855 cm−1 can be assigned to –C–O–C– stretching and –C–C– symmetric stretching, respectively [41].
The chemical structures of the synthesized polyester molecules were confirmed by 1H-NMR analysis. Figure 3 shows the 1H-NMR spectrum for UP04. The peak at 7.4 ppm was assigned to the H atoms of the FDCA furanic ring, while those at 5.9–6.7 ppm were assigned to the H atoms of the IA carbon–carbon double bond. Nuclei bound to C atoms will be more shielded, and thus associated with lower values of the frequency. For these reasons, peaks from 5.5 to 3.8 ppm were attributed to –CH2– groups from diols, and those between 1 and 1.4 ppm to their –CH3 groups.
Two reactive diluents were used as alternatives to styrene for the formulation of the unsaturated polyester resin. BDM was chosen despite the fact that it can be considered only potentially bio-based, as it can be prepared by esterification of 1,4-butanediol and methacrylic acid. Indeed, 1,4-butanediol is easily derived by the reduction of succinic acid, while the production of bio-based methacrylic acid, achieved by the fermentation of sugars, is currently at the R&D stage, although it is envisaged that the commercial process will be available by the end of this decade [13]. DMI was also chosen as a possible alternative to styrene. It is normally obtained by the esterification of IA and bio-methanol. DMI is commercially available and does not have to be synthesized in a research laboratory. Moreover, interesting examples concerning the use of DMI as a reactive diluent for the production of unsaturated polyester resins have already been reported in the literature [27, 42].
All the synthesized polyesters were diluted and crosslinked, as described in the Methods section above. Among the resulting materials, two resins showed a high degree of crosslinking, as indicated by their high gel contents, UP04-BDM (96%) and UP05-DMI (90%) (Table 4). The resin diluted with BDM (UP04-BDM) exhibited a higher gel content and therefore a better crosslinking efficiency than the DMI-based one (UP05-DMI). However, the Tg of UP04-BDM was only around 42 °C, whereas a higher Tg was obtained with the resin diluted in DMI (55 and 75 °C). UP05 diluted with DMI (UP05-DMI) also exhibited sufficiently good results in terms of gel content (90%) and therefore appears to be a promising green candidate for composite manufacturing.
Results of LCA
LCA was performed for three different unsaturated resins:
-
a standard fossil-based resin, i.e., an isophthalic-acid-based unsaturated polyester resin crosslinked with styrene (denoted UPE1);
-
a bio-based polyester resin, prepared with a partially bio-based reactive diluent (UP04-BDM denoted UPE2); and
-
a bio-based polyester resin, prepared with a fully bio-based diluent (UP05-DMI, denoted UPE3).
Figure 4 shows the total impact in terms of GHG emissions and NREU for the three unsaturated polyesters studied (UPE1, UPE2 and UPE3, as defined in Table 1), including the contributions resulting from the choice of monomer, the polymerization process used to produce the binder, and the choice of diluent. The contribution of the different monomers used for the production of the fossil-based polyester is not shown. It can be seen that the use of bio-based monomers and diluents reduced the total GHG emissions significantly, by about 21% for UPE2 and 42% for UPE3, compared to UPE1. Large reductions in the total NREU were also observed, of about 46% and 70% for UPE2 and UPE3, respectively. The polymerization process had a very low contribution to the total cradle-to-factory-gate emissions. The difference between the two bio-based polyesters, UPE2 and UPE3, lies in the diluent used. It can be seen that changing from a partially bio-based diluent BDM (diluent 2), to 100% bio-based diluent DMI (diluent 3), led to a significantly lower impact in terms of both GHG emissions and NREU of about − 27.5% and − 44.3%, respectively.
Sensitivity Analysis
The sensitivity analysis showed significant differences when monomers and diluents obtained from different biomasses were used. Figure 5 shows the total impact on GHG and NREU for the two bio-based UPE options (UPE2 and UPE3).
Considering the change in the production of some monomers, a remarkable modification can be noticed in the total result in terms of both GHG emissions and NREU. Therefore, this life cycle assessment can provide valuable information to guide a scaling process in order to produce high-quality material with desirable environmental performance.
Conclusions
This paper describes the production and characterization of bio-based unsaturated polyester resins as a step towards more sustainable industrial production of glass-fiber-reinforced composites based on a thermosetting matrix for the construction sector. The monomers for polyester synthesis were chosen carefully, focusing on sustainability of the raw materials and their commercial availability. The aim was to replace fossil-based reactive diluents and reactants commonly used for UPE synthesis. IA was chosen as a carrier of unsaturation sites, while 2,5-FDCA was chosen as it was hoped that its rigid structure would enhance the stiffness of the final polyester. Moreover, both 1,2-PD and 1,3-PD were added at various amounts as possible green alternatives to commonly used fossil-based diols. Five different unsaturated polyester resins were prepared and characterized, to identify the optimal properties for bio-based polyesters, mainly in terms of the glass transition temperature. The chemical structure of the final polymers was confirmed via FTIR and 1H-NMR spectroscopy, while their Tg and molecular weights were investigated through DSC and GPC analyses.
The main conclusions drawn from the findings of this study are given below:
-
DSC analysis revealed the positive effect of 1,2-PD in increasing the Tg of the polyester, compared to 1,3-PD. DSC analysis also confirmed the expected effect of FDCA, increasing the stiffness of the polyester structure. Based on these findings, two polyesters with a higher Tg (30 and 32 °C) were identified as the resins with a structure and properties suitable for use with a reactive diluent for crosslinking. Butanediol dimethacrylate (BDM) and dimethyl itaconate (DMI) were used as reactive diluents and were respectively considered to be partially and fully bio-based alternatives to styrene.
-
Unsaturated polyester resin diluted with DMI (UP05) and synthesized using lower reaction temperatures and TTIP as a catalyst, showed a Tg of 75 °C, which was higher than that when the polyester was mixed with BDM (42 °C). Acceptable values of gel content were found in both cases, indicating effective crosslinking of the resins prepared in this fashion.
-
The low environmental impacts of the bio-based polyester resins with a higher Tg, diluted with BDM and DMI (UPE2 and UPE3, respectively) were confirmed by LCA in terms of GHG emission and NREU. The relative calculated category indicator results for the two resins were compared to those of a fossil-based resin (an isophthalic acid-based unsaturated polyester diluted in styrene, UPE1). As expected, the fully bio-based resin, UPE3, showed the lowest environmental impact, followed by UPE2, and then the reference resin, UPE1. However, these results should be interpreted with caution as the sensitivity analysis showed that the result can depend on the selection of information used for the monomer production.
In conclusion, the unsaturated polyester resins described in this study showed promising results in terms of glass transition temperature and degree of crosslinking. Development of these resins will pave the way for the use of completely bio-based renewable sources for the industrial production of thermosetting polyester resins. Future studies will be focused on the further increasing the Tg and the evaluation of the mechanical properties of the resins. The use of transesterification reactions for the synthesis of bio-based polyesters starting from methyl ester derivatives of acids can be explored in the future as well as the effect of different reactive diluents on the degree of crosslinking.
References
Worzakowska M (2009) J Appl Polym Sci 114:720
Kakati N, Assanvo EF, Kalita D (2019) J Polym Environ 27:2540
Johnson KG, Yang LS (2004) Preparation, properties and applications of unsaturated polyesters. In: Scheirs J, Long TE (eds) Modern polyesters: chemistry and technology of polyesters and copolyesters. Wiley, Hoboken, p 697
Gonçalves FAMM, Fonseca AC, Domingos M, Gloria A, Serra AC, Coelho JFJ (2017) Prog Polym Sci 68:1
Hu D, Jia Z, Li J, Zhong B, Fu W, Luo Y, Jia D (2018) J Polym Environ 26:1311
Bagherpour S (2012) Fibre reinforced polyester composites. In: Saleh HED (ed) Polyester. IntechOpen, Rijeka
Mcintyre J (2003) The historical development of polyesters. In: Scheirs J, Long TE (eds) Modern polyesters: chemistry and technology of polyesters and copolyesters. Wiley, Chichester, p 3
Neşer G (2017) Procedia Eng 194:19
Dholakiya B (2012) Unsaturated polyester resin for specialty applications. In: Saleh HEDM (ed) polyester. IntechOpen, Rijeka, p 167
Miyagawa H, Mohanty AK, Burgueño R, Drzal LT, Misra M (2006) Ind Eng Chem Res 45:1014
Matynia T, Worzakowska M, Tarnawski W (2006) J Appl Polym Sci 101:3143
Sanchez EMS, Zavaglia CAC, Felisberti MI (2000) Polymer 41:765
Cousinet S, Ghadban A, Fleury E, Lortie F, Pascault J-P, Portinha D (2015) Eur Polym J 67:539
Wu Y, Li K (2016) J Appl Polym Sci. https://doi.org/10.1002/app.43052
Lima MS, Costa CSMF, Coelho JFJ, Fonseca AC, Serra AC (2018) Green Chem 20:4880
Zia KM, Noreen A, Zuber M, Tabasum S, Mujahid M (2016) Int J Biol Macromol 82:1028
Jasinska L, Koning CE (2010) J Polym Sci A 48:2885
Vilela C, Sousa AF, Fonseca AC, Serra AC, Coelho JFJ, Freire CSR, Silvestre AJD (2014) Polym Chem 5:3119
Lambert S, Wagner M (2017) Chem Soc Rev 46:6855
Ma S, Li T, Liu X, Zhu J (2016) Polym Int 65:164
Kwiatkowska M, Kowalczyk I, Kwiatkowski K, Szymczyk A, Rosłaniec Z (2016) Polymer 99:503
Sousa AF, Matos M, Freire CSR, Silvestre AJD, Coelho JFJ (2013) Polymer 54:513
Sousa AF, Fonseca AC, Serra AC, Freire CSR, Silvestre AJD, Coelho JFJ (2016) Polym Chem 7:1049
Robert T, Friebel S (2016) Green Chem 18:2922
Fonseca AC, Lopes IM, Coelho JFJ, Serra AC (2015) React Funct Polym 97:1
Dai J, Ma S, Teng N, Dai X, Shen X, Wang S, Liu X, Zhu J (2017) Ind Eng Chem Res 56:2650
Panic VV, Seslija SI, Popovic IG, Spasojevic VD, Popovic AR, Nikolic VB, Spasojevic PM (2017) Biomacromol 18:3881
García González MN, Börjesson P, Levi M, Turri S (2018) J Polym Environ 26:3626
Brodin M, Vallejos M, Opedal MT, Area MC, Chinga-Carrasco G (2017) J Clean Prod 162:646
ISO 14040, Environmental management e life cycle assessment e principles and framework, Geneva, Switzerland
ISO 14044, Environmental management e life cycle assessment e requirements and guidelines, Geneva, Switzerland
IPCC Climate Change 2007: Synthesis report. Contribution of working groups I, II and III to the fourth assessment report of the intergovernmental panel on climate change. In: Core Writing Team, Pachauri RK, Reisinger A (eds) IPCC, Geneva, Switzerland, https://ipcc.ch/publications_and_data/ar4/syr/en/contents.html. Accessed 3 July 2019
Nuss P, Gardner KH (2013) Int J Life Cycle Assess 18:603
Montazeri M, Zaimes GG, Khanna V, Eckelman MJ (2016) ACS Sustain Chem Eng 4:6443
Gonzalez-Garay A, Gonzalez-Miquel M, Guillen-Gosalbez G (2017) ACS Sustain Chem Eng 5:5723
Adom F, Dunn JB, Han J, Sather N (2014) Environ Sci Technol 48:14624
Ecoinvent version 3.5. https://www.ecoinvent.org/
Forte A, Zucaro A, Basosi R, Fierro A (2016) Materials 9:563
Yang W, Hu Y, Chen Z, Jiang X, Wang J, Wang R (2012) Fluid Phase Equilib 314:180
Zhang Y, Guo X, Tang P, Xu J (2018) J Chem Eng Data 63:1316
Socrates G (2001) Infrared and Raman characteristic group frequencies: tables and charts. Wiley, Chichester
Fidanovski BZ, Spasojevic PM, Panic VV, Seslija SI, Spasojevic JP, Popovic IG (2018) J Mater Sci 53:4635
Acknowledgements
The authors would like to thank Alessio Caretto, Andres Gonzalez-Garay, Maria Gonzalez-Miquel, and Gonzalo Guillen-Gosalbez and in particular Andres Gonzalez-Garay for the assessment of non-renewable energy use in the case of 1,2-PD. The authors also acknowledge financial support from the European Commission Horizon 2020 programme, for the project entitled “FiberEUse, Large scale demonstration of new circular economy value-chains based on the reuse of end-of-life fiber reinforced composites” (call H2020-CIRC-2016-TwoStage, Project Number H2020-730323-1).
Funding
Open access funding provided by Politecnico di Milano within the CRUI-CARE Agreement. Financial support for the research was provided by European Commission Horizon 2020 programme, for the project entitled “FiberEUse, Large scale demonstration of new circular economy value-chains based on the reuse of end-of-life fiber reinforced composites” (Project Number H2020-730323-1).
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Suriano, R., Gonzalez, M.N.G. & Turri, S. Environmental Profile and Technological Validation of New High-Tg Unsaturated Polyesters from Fully Bio-Based Monomers and Reactive Diluents. J Polym Environ 29, 1122–1133 (2021). https://doi.org/10.1007/s10924-020-01928-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-020-01928-z