Abstract
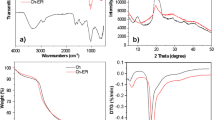
Heavy metal pollution of water sources has become one of the most serious environmental and health problems nowadays. Chitosan (CHI) and derivatives can be used in the complexation and adsorption of heavy metals in water. Hence, this study compared the influence of CHI and carboxymethyl chitosan (CMC) ligands on the competitive complexation between toxic heavy metal ions with opposite charges Cr(VI) and Cd(II) at different pH values as an alternative and ecologically sustainable proposal for industrial spills of heavy metals into aquatic environments. The properties of the synthesized products and CHI were extensively characterized by several spectroscopy techniques, the competitive kinetics of complexation of metal ions with CHI and two type of CMC (with methanol, CMC 40_ME, and with ethanol, CMC 60_ET) was evaluated by wavelength dispersive X-ray fluorescence spectroscopy (WDXRF). The results clearly indicated the influence of the pH and ligands on the competitive complexation of the cationic or anionic ions. The results demonstrated that O-carboxymethylation of chitosan has occurred with a degree of functionalization of (1.20 ± 0.02) and (0.88 ± 0.02) for CMC 40_ME and CMC 60_ET, respectively, leading to the formation of CMC soluble in alkaline medium (pH range of 3.5 ≤ pH ≤ 6.5 and 4.0 ≤ pH ≤ 8.0 for CMC 40_ME and CMC 60_ET, respectively). In alkaline media, complexation of CMC 40_ME is approximately 10% higher (147.2 ± 0.9 mg g−1) than CMC 60_ET (99.8 ± 2.9 mg g−1) with Cd2+ ions at pH 8.5, and approximately 21% lower [(95 ± 2 mg g−1) and (96.3 ± 2.8 mg g−1) for CMC 40_ME and CMC 60_ET, respectively] than CHI (121 ± 6 mg g−1) for CrxO z−y ions at pH 3.0. The kinetic analysis showed variations for each ion and a significant difference regarding the complexant towards the negatively charged ions. The CMC 40_ME, in any analyzed pH, the complexation occurred during the first 45 min of the process. These results showed that CMC, as polydentate functional ligand, was more efficient than CHI especially for the complexation of cations in basic media. Therefore, these systems appear to be attractive alternatives for the containment of industrial spills of heavy metals in wastewater.








Similar content being viewed by others
References
UNESCO (2015) United Nations Educational, Scientific and Cultural Organization Report. UNESCO, Paris
Li X, Li Y, Zhang S, Ye Z (2012) Chem Eng J 183:88–97
Bailey SE, Olin TJ, Bricka RM, Adran DD (1999) Water Res 33:2469–2479
Alakhras F (2019) Arab J Sci Eng 44:279–288
Al-Shahrani H, Alakhras F, Al-Abbad E, Al-Mazaideh G, Hosseini-Bandegharaei A, Ouerfelli N (2018) Glob Nest J 20:620–627
Rojas G, Silva J, Flores JA, Rodriguez A, Ly M, Maldonado H (2005) Sep Purif Technol 44:31–36
Lalvani SB, Wiltowski T, Hubner A, Weston A, Mandich N (1998) Carbon 36:1219–1226
Selvaraj K, Manonmani S, Pattabhi S (2003) Bioresour Technol 89:207–211
Erosa MSD, Medina TIS, Mendoza RN, Rodriguez MA (2001) Hydrometallurgy 61:157–167
Brooks CS (1991) Metal recovery from industrial wastes. Lewis Publishers, Chelsea
Laus R, de Favere VT (2011) Bioresour Technol 102:8769–8776
Monier M (2012) Int J Biol Macromol 50:773–781
Spinelli VA, Laranjeira MCM, Fávere VT (2004) React Funct Polym 61:347–352
Zhang L, Xue J, Zhou X, Fei X, Wang Y, Zhou Y, Zhong L, Han X (2014) Carbohydr Polym 114:514–520
Boamah PO, Huang Y, Hua M, Zhang Q, Liu Y, Onumah J, Wang W, Song Y (2015) Carbohydr Polym 122:255–264
Boamah PO, Huang Y, Hua M, Zhang Q, Wu J, Onumah J, Sam-Amoah LK, Boamah PO (2015) Ecotoxicol Environ Saf 116:113–120
Kyzas GZ, Kostoglou M (2015) Sep Purif Technol 149:92–102
Jiang R, Zhu H, Yao J, Fu Y, Guan Y (2012) Appl Surf Sci 258:3513–3518
Gao C, Liu T, Dang Y, Yu Z, Wang W, Guo J, Zhang X, He G, Zheng H, Yin Y, Kong X (2014) Carbohydr Polym 111:964–970
Pratt DY, Wilson LD, Kozinski JA (2013) J Colloid Interface Sci 395:205–211
Schmitz T, Grabovac V, Palmberger TF, Hoffer MH, Bernkop-Schnurch A (2008) Int J Pharm 347:79–85
Dash M, Chiellini F, Ottenbrite RM, Chiellini E (2011) Prog Polym Sci 36:981–1014
Wang L, Peng Q, Li S, Du L, Cai H (2013) J Ind Eng Chem 19:655–658
Alakhras F, Al-Shahrani H, Al-Abbad E, Al-Rimawi F, Ouerfelli N (2019) Pol J Environ Stud 28:1523–1534
Muzzarelli RAA (2011) Carbohydr Polym 83:1433–1445
Farag RK, Mohamed RR (2012) Molecules 18:190–203
Summer ME (1999) Handbook of soil science, manuals. I. Library of Congress, Section B, Cap. 7 and 8. CRC Press, Boca Raton
Voleski B, Holan ZR (1995) J Biotechnol 11:235–250
Echeverría JC, Morera MT, Maziarán C, Garrido JJ (1998) Environ Pollut 101:275–284
Murali V, Aylmore LAG (1983) Soil Sci 135:143–150
Alakhras F, Ouerfelli N, Al-Mazaideh G, Ababneh T, Al-Abbad E, Abouzeid F (2019) Arab J Sci Eng 44:159–168
Chen X-G, Park H-J (2003) Carbohydr Polym 53:355–359
de Abreu FR, Campana-Filho SP (2009) Carbohydr Polym 75:214–221
Nahalka J, Nahálková J, Gemeiner P, Blanárik P (1998) Biotechnol Lett 20:841–845
de Oliveira-Rosa TR, Debrassi A, da Silva RML, Bressan C, de Freitas RA, Rodrigues CA (2012) J Appl Polym Sci 124:4206–4212
Hasan S, Ghosh TK, Viswanath DS, Boddu VM (2008) J Hazard Mater 152:826–837
Zhao F, Repo E, Yin D, Sillanpaa ME (2013) J Colloid Interface Sci 409:174–182
Roberts GAF (1992) Chitin chemistry. Macmillan, London
Sorlier P, Viton C, Domard A (2002) Biomacromol 3:1336–1342
Yan H, Dai J, Yang Z, Yang H, Cheng R (2011) Chem Eng J 174:586–594
Liu XF, Guan Y, Yang DZ, Li Z, Yao KD (2000) J Appl Polym Sci 79:1324–1335
Franca EF (2009) Biomolecular characterization of biopolymers in solution using computer simulation. PhD Thesis in Chemistry. Center for Science and Technology of Federal University of São Carlos, São Carlos
Delgado AV, Gonzalez-Caballero F, Hunter RJ, Koopal LK, Lyklema J (2005) Pure Appl Chem 77:1753–1805
Tan SC, Khor E, Tan TK, Wong SM (1998) Talanta 45:713–719
Heidari A, Younesi H, Mehraban Z, Heikkinen H (2013) Int J Biol Macromol 61:251–263
Jung C, Heo J, Han J, Her N, Lee S-J, Oh J, Ryu J, Yoon Y (2013) Sep Purif Technol 106:63–71
Acknowledgements
The authors acknowledge the financial support from CNPq, CAPES, FAPEMIG and FINEP/CT-INFRA. The authors express their gratitude to Prof. Luiz Carlos and PhD Student Poliane Chagas (ICEX-UFMG) for the Nuclear Magnetic Resonance analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Borsagli, F.G.L.M., Borsagli, A. Chemically Modified Chitosan Bio-Sorbents for the Competitive Complexation of Heavy Metals Ions: A Potential Model for the Treatment of Wastewaters and Industrial Spills. J Polym Environ 27, 1542–1556 (2019). https://doi.org/10.1007/s10924-019-01449-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-019-01449-4