Abstract
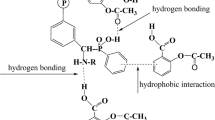
The polyaniline (PANi) film was synthesized by electrochemical polymerization and is used as adsorbent for removal of trimellitic and pyromellitic acids from aqueous solution. The effects of experimental parameters such as: pH, contact time, initial concentration, and temperature were investigated. The optimum adsorption is achieved at pH = 6.5 after 120 min of contact time. The experimental adsorption data were best described by the pseudo-second order model and Langmuir isotherm model. The maximum adsorption capacities of PANi film are of 190.17 and 204.18 mg/g for trimellitic and pyromellitic acids, respectively. The values of thermodynamic parameters indicate that the adsorption is endothermic and spontaneous in nature. The regeneration of the PANi film showed a low reduction (<9 %) in the adsorption efficiency after four cycles of adsorption–desorption. In addition, the quantum calculations using density functional theory at B3LYP/6-31G(d) level confirmed that the adsorption mechanism was a physisorption process with small values of interaction energy between adsorbate and adsorbent. The trimellitic and pyromellitic acids were adsorbed via carbonyl oxygen atoms of their carboxylic groups on the amino group of PANi. The PANi film can be used as a potential, reusable and easily separable adsorbent for removal of aromatic acids from water.









Similar content being viewed by others
References
Samios S, Lekkas T, Nikolaou A, Golfinopoulos S (2007) Desalination 210:125–137
Donald S, Bishop AG, Prenzler PD, Robards K (2004) Anal Chim Acta 527:105–124
Yang Y, Wang T (1997) Vib Spectrosc 14:105–112
Rodríguez FJ, Núñez LA (2011) Water Environ J 25:163–170
Martin JP, Haider K (1971) Soil Sci 111:54–63
Bertilsson S, Tranvik LJ (2000) Limnol Oceanogr 45:753–762
Evanko CR, Dzombak DA (1998) Environ Sci Technol 32:2846–2855
Kumar KV, Shashi A, Surendra A (2003) Carbon 41:765–773
Assabane A, Ait Ichou Y, Tahiri H, Guillard C, Herrmann JM (2000) Appl Catal B 24:71–87
Devi LG, Raju KSA, Kumar SG, Rajashekhar KE (2011) J Taiwan Inst Chem Eng 42:341–349
Bauer C, Jacques P, Kalt A (2001) J Photochem Photobiol A 140:87–92
Galindo C, Jacques P, Kalt A (2001) Chemosphere 45:997–1005
Saleh TA, Gupta VK (2016) Nanomaterial and polymer membranes: synthesis, characterization, and applications. Elsevier, Amsterdam
Laabd M, El Jaouhari A, Ait Haki M, El Jazouli H, Bazzaoui M, Kabli H, Albourine A (2016) J Environ Chem Eng 4:1869–1879
Abdelbassit MSA, Alhooshani KR, Saleh TA (2016) Adv Powder Technol. doi:10.1016/j.apt.2016.06.003
Saleh TA (2016) Desalin Water Treat 57:10730–10744
Saleh TA, Al-Saadi AA (2015) Surf Interface Anal 47:785–792
Chafai H, Laabd M, Elbariji S, Bazzaoui M, Albourine A (2016) J Dispers Sci Technol. doi:10.1080/01932691.2016.1207185
Basar CA (2006) J Hazard Mater 135:232–241
Demirbaş Ö, Alkan M (2013) Desalin Water Treat 53:3623–3631
Chakraborty S, De S, DasGupta S, Basu JK (2005) Chemosphere 58:1079–1086
Ho YS, Chiu WT, Wang CC (2005) Bioresour Technol 96:1285–1291
Anirudhan TS, Aswathy ES, Deepa JR (2016) J Polym Environ. doi:10.1007/s10924-016-0762-y
Zhu L, Wang Y, He T, You L, Shen X (2016) J Polym Environ 24:148–158
Gong R, Feng M, Zhao J, Cai W, Liu L (2009) Bioresour Technol 100:975–978
Ghorbani M, Esfandian H, Taghipour N, Katal R (2010) Desalination 263:279–284
Ali I, Asim M, Khan TA (2012) J Environ Manag 113:170–183
Bhadraa S, Khastgir D, Singhaan NK, Lee JH (2009) Prog Polym Sci 34:783–810
El Jaouhari A, Laabd M, Bazzaoui EA, Albourine A, Martins JI, Wang R, Nagy G, Bazzaoui M (2015) Synth Met 209:11–18
Wang HL, Romero RJ, Mates BR, Zhu Y, Winokur MJ (2000) J Polym Sci B Polym Phys 38:194–204
Laabd M, Ait Ahsaine H, El Jaouhari A, Bakiz B, Bazzaoui M, Ezahri M, Albourine A, Benlhachemi A (2016) J Environ Chem Eng. doi:10.1016/j.jece.2016.06.024
Laabd M, El Jaouhari A, Chafai H, Aarab N, Bazzaoui M, Albourine A (2015) J Mater Environ Sci 6:1049–1059
Ait Haki M, Laabd M, Chafai H, Kabli H, Ez-zahery M, Bazzaoui M, Lakhmiri R, Albourine A (2016) J Dispersion Sci Technol. doi:10.1080/01932691.2016.1184096
Aarab N, Laabd M, Bazzaoui M, Albourine A (2015) J Mater Environ Sci 6:1234–1242
Ganash AA, Al-Nowaiser FM, Al-Thabaiti SA, Hermas AA (2011) Prog Org Coat 72:480–485
Frisch MJ et al (2009) GAUSSIAN 09, Rev. D.01. Gaussian Inc., Wallingford
Becke AD (1993) J Chem Phys 98:5648–5652
Ullah H, Shah AHA, Ayub K, Bilal S (2013) J Phys Chem C 117:4069–4078
Cossi M, Rega N, Scalmani G, Barone V (2003) J Comput Chem 24:669–681
Zhang H, Li H, Zhang F, Wang J, Wang Z, Wang S (2008) J Mater Res 23:2326–2332
Socrates G (1980) Infrared characteristics group frequencies. Wiley, London
Palaniappan S, John A, Amarnath CA, Rao VJ (2004) J Mol Catal A Chem 218:47–53
Gupta VK, Rastogi A (2008) J Hazard Mater 153:759–766
Saleh TA, Muhammad AM, Ali SA (2016) J Colloid Interface Sci 468:324–333
Mahanta D, Madras G, Radhakrishnan S, Patil S (2008) J Phys Chem B 112:10153–10157
Saleh TA (2015) Environ Sci Pollut Res 22:16721–16731
Lagergren S, Svenska BK (1898) Vetenskapsakad Handl 24:1–39
Ho YS, McKay G (1999) Adsorpt Sci Technol 17:233–243
Wu FC, Tseng RL, Juang RS (2001) Environ Technol 22:205–213
Saleh TA (2015) J Water Supply Res Technol AQUA 64:892–903
Langmuir I (1918) J Am Chem Soc 40:1361–1368
Liu C, Bai R, Hong L (2006) J Colloid Interface Sci 303:99–108
Temkin MJ, Pyzhev V (1940) Acta Physiochim USSR 12:217–222
Futalan CM, Kan CC, Dalida ML, Hsien KJ, Pascua C, Wan MW (2011) Carbohydr Polym 83:528–536
Laabd M, El Jaouhari A, Chafai H, Bazzaoui M, Kabli H, Albourine A (2016) Desalin Water Treat 57:15176–15189
Pan BC, Xiong Y, Li AM, Chen JL, Zhang QX, Jin XY (2002) React Funct Polym 53:63–72
Laabd M, Chafai H, Aarab N, El Jaouhari A, Bazzaoui M, Kabli H, El Jazouli H, Albourine A (2016) Environ Chem Lett. doi:10.1007/s10311-016-0569-z
Al-qodah Z (2000) Water Res 34:4295–4303
Doğan M, Alkan M, Turkyılmaz A, Özdemir Y (2004) J Hazard Mater B 109:141–148
Seki Y, Yurdakoç K (2006) Adsorption 12:89–100
Okulik N, Jubert AH (2005) Internet Electron J Mol Des 4:17–30
Ullah H, Shah AHA, Bilal S, Ayub K (2014) J Phys Chem C 118:17819–17830
Monti OLA (2012) J Phys Chem Lett 3:2342–2351
Ullah H, Ayub K, Ullah Z, Hanif M, Nawaz R, Bilal S (2013) Synth Met 172:14–20
Ullah H, Shah AHA, Bilal S, Ayub K (2013) J Phys Chem C 117:23701–23711
Benitex Y, Baranger AM (2011) J Am Chem Soc 133:3687–3689
Saleh TA, Gupta VK, Al-Saadi AA (2013) J Colloid Interface Sci 396:264–269
Bibi S, Ullah H, Ahmad SM, Ali Shah AH, Bilal S, Ali Tahir A, Ayub K (2015) J Phys Chem C 119:15994–16003
Acknowledgments
This work was supported by the MESRSFC and CNRST (Morocco) under Grant No PPR/30/2015.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Laabd, M., El Jaouhari, A., Bazzaoui, M. et al. Adsorption of Benzene-Polycarboxylic Acids on the Electrosynthesized Polyaniline Films: Experimental and DFT Calculation. J Polym Environ 25, 359–369 (2017). https://doi.org/10.1007/s10924-016-0814-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-016-0814-3