Abstract
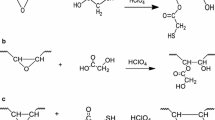
Soybean polyols prepared by ring opening reactions of epoxidized soybean oil with hydrogen active compounds (water, alcohols, organic or inorganic acids, thiols, hydrogen etc.) have a low reactivity in the reaction with isocyanates because the hydroxyl groups are secondary. This paper presents a simple and convenient method to increase the reactivity of soybean polyols with secondary hydroxyl groups by ethoxylation reactions with the preservation of triglyceride ester bonds. The method uses mild reaction conditions: low alkoxylation temperature of 35–45 °C, low pressure of 0.1–0.2 MPa (15–30 p.s.i.) and a superacid as catalyst (HBF4). The new soybean polyols have a higher reactivity toward isocyanates in polyurethane formation due to the high percentage of primary hydroxyl groups. The primary hydroxyl content was determined by the second order kinetics of polyol reaction with phenyl isocyanate.














Similar content being viewed by others
References
White L (2006) Urethanes Technol 23(4):22
Reed D (1997) Urethanes Technol 14(2):20
Ionescu M (2005) Chemistry and technology of polyols for polyurethanes, Rapra Technology Limited (UK), Chapter 17. Polyols from renewable resources. Oleochemical polyols
Guo A, Cho Y, Petrović ZS (2000) J Polym Sci 38:3900
Guo A, Javni I, Petrović ZS (2000) J Appl Polym Sci 77:467
Petrovic ZS, Guo A, Javni I (2000) U.S. Patent 6,107,433
Petrovic ZS, Javni I, Guo A, Zhang W (2002) U.S. Patent 6,433,121
Petrovic ZS, Javni I (2006) WO 2006012344
Petrovic ZS, Javni I (2004) U.S. Patent 6,686,435,2004
Zlatanić A, Petrović ZS, Dušek K (2002) Biomacromolecules 3(5):1048
Herrington R (1997) Flexible polyurethane foams. The Dow Chemical Company, Midland, MI
Hanna IG, Siggia S (1964) J Polym Sci 56:297
Willeboose F, Critchfield FE (1964) Anal Chem 36(12):2270
Kubisa P, Penczek S (1999) Prog Polym Sci 24:1409
Biedron T, Szymanski R, Kubisa P, Penczek S (1990) Makromol Chem Macromol Symp 32:155
Bednarek M, Kubisa P, Penczek S (1989) Makromol Chem Supp1 5:49
Bednarek M, Biedron T, Kubisa P, Penczek S (1991) Makromol Chem Macromol Sym 42/43:475
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ionescu, M., Petrović, Z.S. & Wan, X. Ethoxylated Soybean Polyols for Polyurethanes. J Polym Environ 18, 1–7 (2010). https://doi.org/10.1007/s10924-007-0070-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-007-0070-7