Abstract
Anatomy of bat genital organs has been conventionally studied by gross and microscopic observations to date. Here, we employ both histological observation and diceCT (diffusible iodine-based contrast-enhanced computed tomography) to study the detailed three-dimensional morphological structure of the male genital organs in bats, using the greater horseshoe bat, Rhinolophus ferrumequinum. This is the first study to three-dimensionally describe the whole reproductive organs of bats in detail. Our highly resolved three-dimensional reconstruction reveals that the male organs of R. ferrumequinum consist of paired testes, epididymides, deferent ducts, and five accessory genital glands. The boundary between the ampullary and vesicular glands has been difficult to identify in previous observations, but our diceCT imaging allowed us to clearly differentiate the two. We found that the ampullary gland is located at the terminal part of the deferent ducts, and the vesicular gland lies distal to the ampullary glands. This species possesses a single and carrot-shaped urethral gland, which is not found in most chiropteran families. The presence of the urethral gland in this species and its secretions suggest that after copulation this species is capable of forming a vaginal plug, which can seal the female’s vaginal orifice to block the entrance of spermatozoa from other males. The presence of the urethral gland and elongated epididymal tail and the fact that some individuals can terminate their hibernation and reactivate imply forced copulation on hibernating females can occur in R. ferrumequinum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There are approximately 1300 species of bats (Order Chiroptera) distributed worldwide, from tropical regions to temperate regions (Wilson and Mittermeier 2019). Until a decade ago, bats had been traditionally classified into two suborders, Megachiroptera and Microchiroptera, based on morphological traits (Dobson 1875; Smith 1976; Jones et al. 2002). However, molecular genetic studies in recent decades have shown that Microchiroptera bats are not monophyletic and that members of the Rhinolophoidea superfamily (previously classified as Microchiroptera) are the sister group of Pteropodidae (Jones and Teeling 2006). Thus, the classification has been largely revised as Yinpterochiroptera (for Rhinolophoidea + Megachiroptera) and Yangochiroptera (for non-rhinolophoid microchiropterans)(Springer 2013). Bats have high interspecific variation in their reproductive strategies and variable genital morphology, which reflects the ecological diversity.
The mammalian male reproductive organs comprise paired testes, epididymis, and accessory genital glands, with the testis, epididymis, and accessory genital glands exhibiting variation in shape and position (Miotti et al. 2018; Santos et al. 2018). Four types of testes locations (permanently abdominal, permanently scrotal, migratory, and external) are described, which do not necessarily reflect phylogenetic relationships (Table 1). The testes are located in the abdomen (permanently abdominal testes) in Rhinopomatidae (Rhinopoma kinneari, Anand Kumar 1965; Rhinopoma hardwickei, Karim and Banerjee 1989) and Molossidae (Tadarida aegyptiaca, Bernard and Tsita 1995). In Taphozous longimanus (Krishna 1983) and Nycteridae (Nycteris hispida and Nycteris luteola, Matthews 1942), the testes are found in the scrotum (permanently scrotal). Some bat species show testes migration from the inside to the outside of the abdomen (migratory), depending on the season (Krutzsch 1955; Kitchener 1980; Crichton and Krutzsch 1987). In Taphozous hilli (Kitchener 1980), the testes migrate from the abdomen to the external inguinal ring, and in Mormopterus planiceps (Crichton and Krutzsch 1987) and Tadarida brasiliensis (Krutzsch 1955) the testes migrate to the inguinal canal. Within this migratory type, spermatogenesis may vary depending on the location of the testes. In Tadarida hindei, spermatogenesis only occurs when the testes are outside the abdomen (Marshall and Corbet 1959), whereas in Tadarida condylurus, spermatogenesis can occur both inside and outside the abdomen (Mutere 1973). External testes are the most common in bats. This type can be further divided into three patterns, according to the testes location relative to the penis: lateral to the penis (e.g., Natalus stramineus, Broadbooks 1961; Mitchell 1965, and Rhinolophus megaphyllus, Krutzsch et al. 1992), outside the external inguinal ring (e.g., Thyroptera tricolor and Myzopoda aurita, Krutzsch 2000), and inside the testicular pouch (e.g., Macrotus waterhousii, Krutzsch et al. 1976). The epididymis can be partitioned into three parts: head, body, and tail. Spermatozoa are stored in the tail of the epididymis, and spermatogenesis can take place during a specific season (Mormopterus planiceps, Crichton and Krutzsch 1987) or throughout the year (e.g., Rhinolophus capensis, Bernard 1985, 1986 and Taphozous georgianus, Jolly and Blackshaw 1987). In some hibernating species (e.g., Pipistrellus pipistrellus and Nyctalus noctula), androgenesis and functionality of the accessory genital glands are maintained to preserve the spermatozoa in their epididymal tail during hibernation (Racey and Tam 1974; Racey 1979).
The accessory genital glands can be generally divided into four categories: the ampullary, vesicular, prostate, and bulbourethral glands (König and Liebich 2013; Singh 2017). In humans and dogs, the prostate gland has a simple structure and does not show a multilobed pattern (Prins and Lindgren 2015). However, in rodents, it presents a multilobed pattern divided into dorsal, dorsolateral, and ventral lobes (Price 1963; Jesik et al. 1982; Pinheiro et al. 2003; Rochel et al. 2007). Regarding vesicular glands, they are common to humans, bulls, and rats, but absent in carnivorans (Setchell and Breed 2006; Prins and Lindgren 2015). The bulbourethral glands are present in boars, but are poorly developed in humans and not found in dogs (Jequier 1995; Badia et al. 2006).
Previous gross and histological observations have shown that the accessory genital glands of bats can be defined as ampullary, vesicular, prostate, urethral, and bulbourethral (Krutzsch 2000). The prostate and bulbourethral glands have been found in all bat species studied to date (Krutzsch 2000). The prostate gland, in particular, shows remarkable interspecific variation and can be further separated into two (e.g., Artibeus planirostris, Puga et al. 2012; Desmodus rotundus and Platyrrhinus lineatus, Martins et al. 2015; Noctilio albiventris and Rhynchonycteris naso, Beguelini et al. 2016; Sturnira erythromos, Sturnira lilium, and Sturnira oporaphilum, Miotti et al. 2018; Artibeus lituratus, Santos et al. 2018) or three compartments (e.g., Myotis nigricans, Negrin et al. 2014; Molossus molossus, Christante et al. 2015; Carollia perspicillata, Glossophaga soricina, and Phyllostomus discolor, Martins et al. 2015; Martins et al. 2016) (Table 1). The ampullary glands are present in most bat species (Krutzsch 2000), such as Rhinopomatidae (Karim and Banerjee 1989), Emballonuridae (Beguelini et al. 2016), Natalidae, Nycteridae (Krutzsch 1979), Megadermatidae (Fard and Ghassemi 2017), Rhinolophidae (Krutzsch et al. 1992), Vespertilionidae (Gadegone and Sapkal 1983), and Molossidae (Crichton and Krutzsch 1987); whereas Pteropodidae (Danmaigoro et al. 2014) and Phyllostomatidae (Krutzsch 1979) do not have them. The presence of vesicular glands is highly variable among bats. Pipistrellus hesperus (Krutzsch 1975), Pipistrellus dormer (Gadegone and Sapkal 1983), Mormopterus planiceps (Crichton and Krutzsch 1987), and Miniopterus schreibersii have them (Krutzsch and Crichton 1990), while Hipposideros fulvus (Patil 1968), Thyroptera tricolor (Wimsatt and Enders 1980), Hipposideros speoris (Pal 1983), Scotophilus heathi (Krishna and Singh 1997) and Rhinolophus capensis do not (Bernard 1985). It can also be found in Pteropodidae, Emballonuridae, Phyllostomatidae, Vespertilionidae, and Molossidae, while being absent in Rhinopomatidae, Noctilionidae, and Natalidae (Krutzsch 2000). The urethral gland is only found in Rhinolophus (Krutzsch 2000).
Anatomy of bat genital organs has been studied mainly by gross and microscopic observations to date. However, these inevitably disrupt the intact three-dimensional structure, making it difficult to compare the subtle and overlooked anatomical differences among species. The accessory genital glands, in particular, have been very difficult to observe using conventional techniques. Recently, microcomputed tomography (microCT) imaging has demonstrated tremendous potential in unraveling previously undescribed bat anatomy, mainly the penis and baculum (Herdina et al. 2010, 2015a, b), turbinal morphology(Curtis and Simmons 2017), vomeronasal and olfactory system (Yohe et al. 2018), and prenatal cranial bone development (Nojiri et al. 2018). Contrast enhancement methods (diceCT) by iodine staining, in particular, have opened up new avenues in the study of bony structures and soft tissues in detail using X-ray (Gignac and Kley 2014; Gignac et al. 2016).
In this study, we used both histological observations and diceCT to study the detailed morphological structure of male genital organs of bats in 3D for the first time, using the greater horseshoe bat, Rhinolophus ferrumequinum. Genus Rhinolophus belongs to the family Rhinolophidae, suborder Yinpterochiroptera, and R. ferrumequinum is distributed across the Palearctic region from Europe to Japan (Csorba et al. 2003). They have a polygynous mating system, and parturition occurs in summer (Kuramoto 1977; Mori et al. 1982; Rossiter et al. 2000b; Sano 2001). Spermatozoa are produced during the summer and stored in the tail part of the epididymis. Mating occurs in the following autumn (Courrier 1927; Oxberry 1979; Oh et al. 1985a). After mating, the spermatozoa are stored in the female reproductive organs—oviduct, utero-tubal junction, or uterus—through the winter without fertilization (Gustafson 1979; Racey 1979; Mori et al. 1982). Fertilization occurs after they terminate their hibernation (delayed fertilization) (Matthews 1937). Although Rhinolophus is a large specious group in bats, the anatomy of its male genital organs, which is highly diverse, is still poorly described. As noted earlier, Rhinolophus possesses a urethral gland, which is not found in other bats (Krutzsch 2000). Rhinolophus hipposideros (Gaisler 1966) and R. megaphyllus (Krutzsch et al. 1992) have ampullary, vesicular, prostate, urethral and bulbourethral glands, while R. capensis is equipped only with ampullary glands (Bernard 1985). In the case of R. landeri, testes are reported to reach maximum size in the winter and shrink in the summer (Menzies 1973). Regarding R. ferrumequinum, only brief descriptions by Krutzsch (2000) are available, who reported on the structures of the testis and epididymis, although the anatomy of the accessory genital glands were not described in detail. Given these, we present the detailed three-dimensional structure of the male soft-tissue genital organs of bats for the first time, with special reference to the accessory genital glands, and compared those with other bat species to provide insights to the phylogenetic and ecological patterns of the genital organs.
Materials and Methods
We used the genital organs of a fully mature, male great horseshoe bat, obtained on 29 June 2018 in Kiyotsu-kyo, Tokamachi city, Niigata Prefecture, Japan, under the capture permit and ethical approval from the Tokamachi City Government (Permission number 10KAN-63-1-3). The bat was euthanatized by isoflurane overdose. The organs were fixed with 10% formaldehyde for 48 h, transferred to 70% ethanol, and then stained with 1% iodine in ethanol for 14 days before scanning (diceCT). The specimen was first observed macroscopically, and then scanned using a microCT system (inspeXio SMX-90CT Plus, Shimadzu Corp., Kyoto, Japan) with a 90kv source voltage and 100 mA source current; and the voxel size of the images was 34 μm. We reconstructed the serial images of male genital organs using the Amira 5.2 software (Visage Imaging, San Diego, USA). After scanning the genital organs, the sample was used for histological observation. The tissue sample of the genitals was dehydrated with a graded series of ethanol (70–100%), cleared in xylene, and embedded in paraffin. The paraffin-embedded tissue were serially divided into 3-μm-thick sections using a microtome (Reichert-Jung 2040, Leica Corp., Watzlar, Germany), and placed on glass slides (Microscope Slides #1000612, Marienfeld Corp., Lauda-Königshofen, Germany). Deparaffinized sections were stained with hematoxylin and eosin (HE). After staining, the sections were dehydrated in graded series of ethanol, cleared in xylene, and covered with cover glass. Images of all genital organs were captured using a light microscope (BX51, Olympus Corp., Tokyo, Japan) equipped with a digital camera (DP71, Olympus Corp., Tokyo, Japan) connected to a computer. The reconstructed 3D surface STL model generated and analyzed during the current study is available in the MorphoMuseum repository (https://doi.org/10.18563/journal.m3.113). All other datasets analyzed during the current study are available from the corresponding author on request.
Results
Gross Anatomy
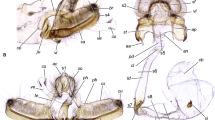
Using macroscopic observation, we confirmed that the male genital organs of R. ferrumequinum comprise five accessory genital glands, paired testes, epididymides, and deferent ducts (Fig. 1). The testis, epididymis, and part of the deferent duct were covered by the scrotum. The testes were located outside the abdomen. The epididymis was separated into three parts—head, body, and tail—which were attached to different regions of the testis. The head part starts on the middle region and medial surface of the testis, and continues to the body part as the epididymal duct, which runs to the tail along the medial surface of the testis. The tail of the epididymis showed a caudal elongation and a recurrently ascending turn into the testis (Fig. 2). Finally, the epididymal duct from the tail part continues as the deferent duct. The accessory genital glands included the ampullary, vesicular, prostate, urethral, and bulbourethral glands. The ampullary glands showed a thickening of the terminal part of the deferent duct, forming the ampulla of the deferent duct. They exhibited cone-shaped bodies, which were located dorsal to the urinary bladder. The vesicular glands lie distal to the ampullary glands, and were presented in a pair, rounded, and bilaterally symmetric in shape. The prostate gland was located dorsodistally to the complex of the ampullary-vesicular glands, surrounding the urethra and continuing to the urethral gland. The urethral gland was carrot-shaped, and encircling the urethra. The bulbourethral glands were located dorsal to the urethral gland, bulbospongious muscle, and both sides of the rectum.
Macroscopic observations of the genital organs in male greater horseshoe bat after removing the skin, muscles and other organs. a dorsal view. b ventral view. Ag, ampullary glands; Bg, bulbourethral glands; Bm, bulbospongious muscle; Dd, deferent duct; Ep, epididymis; Pe, penis; Pg, prostate gland; Te, testis; Ub, urinary bladder; Ug, urethral gland; Vg, vesicular glands. Scale bar: 1 cm
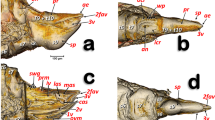
The 3D model reconstructed from diceCT images (Fig. 3) clarified, with precision, the shape, location, and the internal structure of the genital organs. The urethra started from the urinary bladder, and the deferent ducts were joined at the prostate gland. After joining, the duct passed through the inside the urethral gland and reached the tip of the penis. In the penis, the corpus cavernosum formed the greater part of its inside, and the terminal of the urethra was capped by a small, bony ossicle—the os penis. The end of corpus cavernosum and the origin of os penis were connected.
The genital organs of male greater horseshoe bat. a Three-dimensional reconstruction, positions of organs in the pelvic region before removing of the skin, muscle, and other organs. b Position of genital organs, all organs are marked in different colors (skin, muscle and other organs removed). c Dorsolateral view and d dorsal view with penis removed. The os penis, urethra, and corpus cavernosum are located inside the penis. Ag, ampullary glands; Bg, bulbourethral glands; Bm, bulbospongious muscle; Cc, corpus cavernosum; Dd, deferent duct; Ep, epididymis; Op, os penis; Pe, penis; Pg, prostate gland; Te, testis; Ub, urinary bladder; Ug, urethral gland; Ur, urethra; Vg, vesicular glands
Histological Observations
Histological observations of the male reproductive organs were conducted on six locations (the lines of a-g), as shown in Fig. 4. The terminal part of the deferent duct continued to the ampullary gland. The deferent duct was composed of a thick muscular wall. The acini of the ampullary glands were lined with pseudostratified epithelial cells with tall or round nuclei (Fig. 4b). The vesicular glands had an outer layer of connective tissue, and consisted of multiple acini lined with the cuboidal epithelium with round nuclei (Fig. 4c). The prostate gland was lined with pseudostratified epithelium, which can be categorized as transitional epithelium. The prostatic urethra running inside the prostate gland was also lined with pseudostratified epithelium. Prostate gland and prostatic urethra were surrounded by fibromuscular tissue, acting as a support frame (Fig. 4d). The outer layer of the urethral gland was surrounded by a muscular capsule and the acini were lined with simple columnar epithelium without the fibromuscular tissue in the interstitial space (Fig. 4e). The outer layer of the bulbourethral gland consisted of striated muscular tissue (Fig. 4f). The acini were lined with tall columnar epithelial cells with rounded or pyramidal shaped nuclei, located on the basement membrane. Luminal secretion was observed in all glands except the urethral gland. The penis was divided into two sections: the body and the penis glans. The corpus cavernosum was found in the body part and os penis was situated in the penis glans. The terminal part of the corpus cavernosum was connected to the os penis (Fig. 4g) and the corpus cavernosum was composed of smooth muscles and elastic fibers, both of which were surrounded by the tunica albuginea. The paraurethral glands (glands of Littre) were not found in this species (Fig. 5).
Three-dimensional reconstruction of the genital organs for histological observations. a Histological observations were conducted at six locations. b Ampullary gland. The acinus was lined with pseudostratified epithelium (arrows). c Vesicular glands. The acinus was lined with simple columnar epithelium (arrows). d Prostate gland. The acinus was lined by stratified (arrow) or pseudostratified (arrowhead) columnar epithelium. The fibromuscular tissue (M) was present as supporting connective tissue. e Urethral gland. The acinus was lined with simple columnar epithelium (arrows). f Bulbourethral glands. The striated muscle (M) surrounded the gland. The epithelium of acinus was lined with tall columnar cells with round (arrow) or pyramid-shaped (arrowhead) nuclei on the base. The luminal secretions (L) were present in all glands, except the urethral gland. g Penis. The terminal part of the corpus cavernosum was connected to the os penis (arrowheads). The urethra was surround by the corpus spongiosum and lied above the corpus cavernosum. Ag, ampullary glands; Bg, bulbourethral glands; Cc, corpus cavernosum; Cs, corpus spongiosum; Dd, deferent duct; Ep, epididymis; Pe, penis; Pg, prostate gland; Te, testis; Ub, urinary bladder; Ug, urethral gland; Ur, urethra; Vg, vesicular glands
Discussion
We found that the male genital organs of R. ferrumequinum are composed of the paired testes, epididymides, deferent ducts, and accessory genital glands, consistent with the cases reported for R. hipposideros (Gaisler 1966) and R. megaphyllus (Krutzsch et al. 1992). From the root to the shaft of the penis, the corpus cavernosum lies dorsal to the urethra. In the corpus cavernosum, there are blood vessels, smooth muscles, and elastic fibers responsible for erection. The os penis is found inside the penis glans. The epithelium of the urethra is lined by transitional epithelium, suggesting its capability of detention and contraction (Eurell and Frappier 2006).
Among the four types (permanently abdominal, permanently scrotal, migratory, and external), we confirmed that the testes location of R. ferrumequinum can be classified as the external type, the most common pattern found in bats (Table 1). Its testes is located lateral to the penis, inside the scrotum, and the testes are found under the skin, covering the inguinal part. The location of the testes influences spermatogenesis, as it is affected by body temperature and external temperature (Jolly and Blackshaw 1988). In hibernating species with testes permanently located within the abdominal cavity (e.g., Rhinopoma hardwickei, Karim and Banerjee 1989; Rhinopoma kinneari, Anand Kumar 1965), spermatogenesis is more active in the winter, when hibernation takes place. On the contrary, in hibernating species with testes located outside the abdominal cavity (external type), spermatogenesis does not occur during hibernation periods (Lee 2018). Among Rhinolophus, the activation of spermatogenesis is reported to occur during the non-hibernating period in R. cornutus (August and October: Kurohmaru et al. 2002) and R. capensis (October to November: Bernard 1985). The external location of the testes in R. ferrumequinum implies that spermatogenesis does not occur during hibernation periods. Consistent with this prediction, it was reported that spermatogenesis takes place only during active seasons and does not occur during hibernation in Korean (Lee 2018) and Japanese populations (Oh et al. 1985b).
The tail part of the epididymis of R. ferrumequinum is elongated as in R. capensis (Bernard 1985). It is known that the tail of the epididymis is elongated in hibernating bats, such as Pipistrellus kuhlii (Sharifi et al. 2004), Myotis daubentonii (Encarnação et al. 2004), and Neoromicia nanus (van der Merwe and Stirnemann 2007). In addition, the tail of the epididymis is elongated in non-hibernating species, as seen in Emballonuridae (Rhynchonycteris naso) and Vespertilionidae (Eptesicus furinalis, Histiotus velatus, Lasiurus blossevilli, Myotis albescens, and Myotis nigricans) (Beguelini et al. 2012). According to Beguelini et al. (2012), bat species with smaller testis tend to have an elongated epididymis tail, while those with larger testis are likely to show relatively shorter epididymis tails. Elongation of the tail part of the epididymis is, arguably, related to increased sperm storage (Beguelini et al. 2012).
The accessory genital glands of this species consist of the ampullary, vesicular, prostate, urethral, and bulbourethral glands. One of the accessory glands, the ampullary glands, is the terminal part of the deferent duct, continuing to the vesicular glands. Previous studies on other bat species, such as Taphozous georgianus (Jolly and Blackshow 1987), Tadarida brasiliensis mexicana (Krutzsch et al. 2002), point out that the ampullary and vesicular glands are indistinguishable by conventional macroscopic observations, and detailed descriptions have been lacking (Table 1). However, our microCT imaging allowed us to clearly identify and differentiate the two. Additionally, the epithelium of these two glands appears to be clearly differentiated. The epithelium of the ampullary gland is lined with pseudostratified epithelium, which consists of tall columnar cells with ovoid nuclei and cuboidal cells with round nuclei. The apical surface of epithelial cells shows protrusions, suggesting a secretion-related role (Eurell and Frappier 2006). The acini of the vesicular glands are lined with simple cuboidal cells and the lumen is filled with secretions. The vesicular gland secretion generally functions as an energy source for stored spermatozoa (Eurell and Frappier 2006).
The prostate gland is located dorsodistally to the complex of the ampullary-vesicular glands, continuing to the urethral gland. In other species, the prostate is separated into either two (Artibeus planirostris, Puga et al. 2012; Molossus molossus, Christante et al. 2015; Desmodus rotundus and Platyrrhinus lineatus, Martins et al. 2015; Noctilio albiventris and Rhynchonycteris naso, Beguelini et al. 2016; Sturnira erythromos, Sturnira lilium, and Sturnira oporaphilum, Miotti et al. 2018; Artibeus lituratus, Santos et al. 2018) or three portions (Myotis nigricans, Negrin et al. 2014; Carollia perspicillata, Glossophaga soricina, and Phyllostomus discolor, Martins et al. 2016)(Table 1). In contrast, we found that this gland constitutes a single structure in R. ferrumequinum (Fig. 3). The prostate was circular, and surrounding the urethra. Generally, in mammals, the prostate gland is single lobed, and its secretion is serous or seromucous (Eurell and Frappier 2006). The role of this secretion is, primarily, to activate ejaculated spermatozoa and neutralize the seminal plasma (Eurell and Frappier 2006), although the functional significance of lobe numbers is unclear and should be addressed in future research.
We confirmed that, in R. ferrumequinum, the bulbourethral glands are located dorsal to the urethral gland and bulbospongious muscle, and bilaterally to the rectum. The bulbourethral glands consist of smooth and striated muscle fibers and the epithelium is lined with tall columnar cells. The ability of luminal secretion was confirmed in the bulbourethral glands, although the type of secretions was undetectable in this study. While the shape of bulbourethral glands can vary from punching bag (e.g., Macrotus waterhousii, Krutzsch et al. 1976), to oval (e.g., R. megaphyllus, Krutzsch et al. 1992), or teardrop-shaped (e.g., Molossus molossus, Christante et al. 2015; Artibeus lituratus, Santos et al. 2018), in R. ferrumequinum, it was oval. Most bats including, R. ferrumequinum, exhibit one pair of bulbourethral glands, except for Corynorhinus rafinesquii, which possesses two pairs of this gland (Pearson et al. 1952). According to Krutzsch (2000), the bulbourethral glands are generally located bilaterally to the rectum, anus, or near the base of the penis. In R. ferrumequinum, the duct of the bulbourethral glands is found on the bulbospongious muscle and is connected to the urethra, which is beneath this muscle. We confirmed that such topology is virtually similar to that of R. megaphyllus (Krutzsch et al. 1992).
The urethral gland in this species is a well-developed, carrot-shaped single structure, surrounded by a muscular capsule. The epithelium of the gland is simple columnar epithelium; however, no glandular luminal secretion is found. Among bats, the urethral gland is reportedly found only in Rhinolophidae (Hipposideros caffer, Matthews 1942; R. hipposideros, Gaisler 1966; R. capensis, Bernard 1985; R. megaphyllus, Krutzsch et al. 1992) and Megadermatidae (Cardioderma cor, Matthews 1942), all of which belong to Yinpterochiroptera. It has been reported that, generally, the prostate gland is divided into multiple lobes, and the urethra that penetrates the prostate gland is surrounded by the paraurethral glands (e.g., Desmodus rotundus, Platyrrhinus lineatus, Carollia perspicillata, Glossophaga soricina, and Phyllostomus discolor, Martins et al. 2015; Artibeus planirostris, Puga et al. 2012; Molossus molossus, Christante et al. 2015)(Table 1). Although the paraurethral glands are also (occasionally) referred to as glands of Littre, or “urethral gland” (Beguelini et al. 2016), this should not be confused with the urethral gland described here. While the paraurethral glands lie in the mucosal and muscular wall stroma of the urethra, and are located between the prostate and prostatic urethra, the urethral gland is located outside the prostate gland (Krutzsch 2000), as seen in R. ferrumequinum. We confirmed that R. ferrumequinum does not possess paraurethral glands (Fig. 5c). Phylogenetic distribution of the urethral and paraurethral glands is still largely unknown and requires further research.
The size of the accessory genital glands, including the urethral gland, varies depending on the season, because the amount of secretions in the lumen corresponds to the reproductive cycle (Racey and Tam 1974; Bernard 1985; Krutzsch et al. 1992). The urethral gland of the R. hipposideros is non-functional for about five months, around spring and summer (Gaisler 1966). In this species, a volume increase is observed from late summer onwards, peaking in autumn. During the winter, the gland enters a resting period and its secretion is arrested, making the lumen practically empty. It must be pointed out that the secretion from this gland forms a vaginal plug that seals the vaginal orifice, preventing sperm loss and blocking the entrance of sperm from other males (Matthews 1937; Gaisler and Titlbach 1964; Gaisler 1966; Oh et al. 1983; Uchida 1987; Lee in press). Similar plugs are known to exist in other mammals, such as rodents, lipotyphlans, and marsupials (Austin and Short 1972; Rochel et al. 2007; Martin et al. 2011). It has been reported that R. ferrumequinum can interrupt hibernation (Sano 2001; Kim et al. 2019). In the Japanese R. cornutus species, evidence of forced mating during hibernation periods has been recently reported, and the functional significance of the vaginal plug for deterring other males during hibernation period has been pointed out (Sato 2019). Thus, the urethral gland’s capability of forming a firm vaginal plug is highly important for successful reproduction (Rossiter et al. 2000a). Whether R. ferrumequinum also engages in forced mating during hibernation is yet to be studied; however, the elongated epididymal tail in R. ferrumequinum suggests that an extended storage of spermatozoa during hibernation period takes place, and supports the possibility of such behavior in this species. Evaluating the occurrence of secretion in the accessory genital glands during the hibernation period is required, in order to confirm the possibility of extended storage of active spermatozoa, amid hibernation and forced mating.
Conclusion
In this study, we performed the first microCT study of the soft-tissue male reproductive organs in bats, providing the first detailed three-dimensional description of the whole structure of the male genital system in bats, using R. ferrumequinum. Until recently, observing and describing the intact accurate structure and composition of accessory genital glands was difficult, due to technical limitations. By using microCT imaging, we successfully described the detailed anatomy of the accessory genital glands in R. ferrumequinum. We concluded that the male reproductive organs of R. ferrumequinum comprise paired testes, epididymides, deferent ducts, and five accessory genital glands. The testes were located external to the abdomen and lateral to the penis, which is the hallmark of hibernating species. The epididymis is attached to the testis, and its tail part showed a caudal elongation with a characteristic turnback to the testis. The ampullary gland is located at the terminal part of the deferent ducts, and the vesicular gland lies distal to the ampullary glands. These two glands have been indistinguishable by conventional macroscopic observations, but our microCT imaging allowed us to identify their boundaries and confirm their anatomical differentiation. The presence of the urethral gland and its secretions strongly supports that this species is capable of forming a vaginal plug. Elongation of the tail part of the epididymis suggests increased sperm storage. Given that some individuals are known to halt their hibernation and awaken, it is possible that forced copulation on hibernating females may occur. Studies on secretion status of the vesicular and prostate glands during hibernation period are required to test this hypothesis.
As most of the previous studies on bat genital organs were conducted before the phylogenetic relationships of bats had been clarified, mapping the diversity of the reproductive organs onto the bat phylogeny and clarifying the evolutionary history of the reproductive organs has yet to be done. Therefore, we envision to further investigate the three-dimensional morphological structures of bat genital organs and clarify the diversity and patterns of the genital organs in bats.
References
Anand Kumar TC (1965) Reproduction in the rat-tailed bat Rhinopoma kinneari. Proc Zool Soc Lond 147:147–155. https://doi.org/10.1111/j.1469-7998.1965.tb04639.x
Austin CR, Short RV (1972) Reproduction in Mammals: Volume 1, Germ Cells and Fertilization. Cambridge University Press, Cambridge
Badia E, Briz MD, Pinart E, Sancho S, Garcia N, Bassols J, Pruneda A, Bussalleu E, Yeste M, Casas I, Bonet S (2006) Structural and ultrastructural features of boar bulbourethral glands. Tissue Cell 38:7–18. https://doi.org/10.1016/j.tice.2005.09.004
Beguelini MR, Puga CCI, Martins FF, Betoli AHS, Taboga SR, Morielle-Versute E (2012) Morphological variation of primary reproductive structures in males of five families of Neotropical bats. Anat Rec 296:156–167. https://doi.org/10.1002/ar.22613
Beguelini MR, Puga CCI, Morielle-Versute E, Taboga SR (2016) Comparative analysis of the male reproductive accessory glands of bats Noctilio albiventris (Noctilionidae) and Rhynchonycteris naso (Emballonuridae). J Morphol 277:1459–1468. https://doi.org/10.1002/jmor.20587
Bernard RTF (1985) Reproduction in the Cape horseshoe bat (Rhinolophus capensis) from South Africa. S Afr J Zool 20:129–135. https://doi.org/10.1080/02541858.1985.11447925
Bernard RTF (1986) Seasonal changes in plasma testosterone concentrations and leydig cell and accessory gland activity in the Cape horseshoe bat (Rhinolophus capensis). J Reprod Fertil 78:413–422. https://doi.org/10.1530/jrf.0.0780413
Bernard RTF, Tsita JN (1995) Seasonally monoestrous reproduction in the molossid bat, Tadarida aegyptiaca from low temperate latitudes (33°S) in South Africa. S Afr J Zool 30:18–22. https://doi.org/10.1080/02541858.1995.11448366
Broadbooks HE (1961) The funnel-eared bat in Sonora. J Mammal 42:403
Christante CM, Beguelini MR, Puga CCI, Negrin AC, Morielle-Versute E, Vilamaior PSL, Taboga SR (2015) Structure, histochemistry and seasonal variations of the male reproductive accessory glands in the Pallas’s mastiff bat, Molossus molossus (Chiroptera: Molossidae). Reprod Fertil Dev 27:313. https://doi.org/10.1071/RD13232
Courrier R (1927) Etude sur le déterminisme des caractères sexuels secondaires chez quelques mammifères à activité testiculaire périodique. Arch Oral Biol 37:173–334
Crichton EG, Krutzsch PH (1987) Reproductive biology of the female little mastiff bat, Mormopterus planiceps (Chiroptera: Molossidae) in Southeast Australia. Am J Anat 178:369–386. https://doi.org/10.1002/aja.1001780408
Csorba G, Ujhelyi P, Thomas N (2003) Horseshoe Bats of the World:(Chiroptera: Rhinolophidae). Alana Books, Shropshire
Curtis AA, Simmons NB (2017) Unique turbinal morphology in horseshoe bats (Chiroptera: Rhinolophidae). Anat Rec 300:309–325. https://doi.org/10.1002/ar.23516
Danmaigoro A, Onu JE, Sonfada ML, Umaru MA, Oyelowo FO (2014) Histology and histometric anatomy of the male reproductive system of bat (Eidolon helvum). J Histol 2014:1–6. https://doi.org/10.1155/2014/834735
Dobson GE (1875) XLVII.—Conspectus of the suborders, families, and genera of Chiroptera arranged according to their natural affinities. Ann Mag Nat Hist 16:345–357
Encarnação JA, Dietz M, Kierdorf U (2004) Reproductive condition and activity pattern of male Daubenton’s bats (Myotis daubentonii) in the summer habitat. Mammal Biol 69:163–172. https://doi.org/10.1078/1616-5047-00131
Eurell JA, Frappier BL (2006) Dellmann’s textbook of veterinary histology. John Wiley & Sons, New York
Fard EA, Ghassemi F (2017) Histological and morophometrical study of male reproductive tract in Rousettus aegyptiacus (Mammalia : Megachiroptera) in Iran. J Entomol Zool Stud 5:229–234
Gadegone MM, Sapkal VM (1983) Mucins in the male accessory sex glands of pipistrellid bat, Pipistrellus Dormeri (Dobson). Comp Physiol Ecol 8:219–222
Gaisler J (1966) Reproduction in the lesser horseshoe bat (Rhinolophus hipposideros hipposideros Bechstein, 1800). Bijdr tot Dierkd 36:45–62
Gaisler J, Titlbach M (1964) The male sexual cycle in the lesser horseshoe bat (Rhinolophus hipposideros hipposideros). Vestn Ceskoslov Spol Zool 28:268–277
Gignac PM, Kley NJ (2014) Iodine-enhanced micro-CT imaging: methodological refinements for the study of the soft-tissue anatomy of post-embryonic vertebrates. J Exp Zool Part B Mol Dev Evol 322:166–176. https://doi.org/10.1002/jez.b.22561
Gignac PM, Kley NJ, Clarke JA, Colbert MW, Morhardt AC, Cerio D, Cost IN, Cox PG, Daza JD, Early CM, Echols MS, Henkelman RM, Herdina AN, Holliday CM, Li Z, Mahlow K, Merchant S, Müller J, Orsbon CP, Paluh DJ, Thies ML, Tsai HP, Witmer LM (2016) Diffusible iodine-based contrast-enhanced computed tomography (diceCT): an emerging tool for rapid, high-resolution, 3-D imaging of metazoan soft tissues. J Anat 228:889–909. https://doi.org/10.1111/joa.12449
Gustafson AW (1979) Male reproductive patterns in hibernating bats. Reproduction 56:317–331. https://doi.org/10.1530/jrf.0.0560317
Herdina AN, Herzig-Straschil B, Hilgers H, Metscher BD, Plenk H Jr (2010) Histomorphology of the penis bone (baculum) in the gray long-eared bat Plecotus austriacus (Chiroptera, Vespertilionidae). Anat Rec 293:1248–1258. https://doi.org/10.1002/ar.21148
Herdina AN, Kelly DA, Jahelková H, Lina PHC, Horáček I, Metscher BD (2015a) Testing hypotheses of bat baculum function with 3D models derived from microCT. J Anat 226:229–235. https://doi.org/10.1111/joa.12274
Herdina AN, Plenk H Jr, Benda P, Lina PHC, Herzig-Straschil B, Hilgers H, Metscher BD (2015b) Correlative 3D-imaging of Pipistrellus penis micromorphology: validating quantitative microCT images with undecalcified serial ground section histomorphology. J Morphol 276:695–706. https://doi.org/10.1002/jmor.20372
Jequier AM (1995) Clinical disorders affecting semen quality. In: Yovich JI (ed) Gametes-The Spermatozoon. Cambridge University Press, Cambridge, pp 175–191
Jesik CJ, Holland JM, Lee C (1982) An anatomic and histologic study of the rat prostate. Prostate 3:81–97. https://doi.org/10.1002/pros.2990030111
Jolly SE, Blackshaw AW (1987) Prolonged epididymal sperm storage, and the temporal dissociation of testicular and accessory gland activity in the common sheath-tail bat, Taphozous georgianus, of tropical Australia. J Reprod Fertil 81:205–211. https://doi.org/10.1530/jrf.0.0810205
Jolly SE, Blackshaw AW (1988) Testicular migration, spermatogenesis, temperature regulation and environment of the sheath-tail bat, Taphozous georgianus. J Reprod Fertil 84:447–455. https://doi.org/10.1530/jrf.0.0840447
Jones G, Teeling EC (2006) The evolution of echolocation in bats. Trends Ecol Evol 21:149–156. https://doi.org/10.1016/J.TREE.2006.01.001
Jones KE, Purvis A, Maclarnon ANN, Bininda-Emonds ORP, Simmons NB (2002) A phylogenetic supertree of the bats (Mammalia: Chiroptera). Biol Rev 77:223–259. https://doi.org/10.1017/S1464793101005899
Karim KB, Banerjee S (1989) Reproduction in the Indian mouse-tailed bat, Rhinopoma hardwickei hardwickei (Chiroptera, Rhinopomatidae). Reprod Fertil Dev 1:255–264. https://doi.org/10.1071/RD9890255
Kim SS, Choi YS, Yoo JC (2019) Regional differences in winter activity of hibernating greater horseshoe bats (Rhinolophus ferrumequinum) from Korea. J Ecol Environ 43:1–8. https://doi.org/10.1186/s41610-018-0097-9
Kitchener DJ (1980) Taphozous hilli sp. nov.(Chiroptera: Emballonuridae), a new sheath-tailed bat from Western Australia and Northern Territory. Rec West Aust Mus 8:161–169
König HE, Liebich H-G (2013) Veterinary Anatomy of Domestic Mammals: Textbook and Colour Atlas. Schattauer Verlag, Stuttgart
Krishna A (1983) Growth of young and sexual maturity in three species of Indian bats. J Anim Morphol Physiol 30:162–168
Krishna A, Singh K (1997) The relationship between testicular activity, accessory sex glands, and circulating steroid concentration during the reproductive cycle in a male Indian vespertilionid bat, Scotophilus heathi. Can J Zool 75:1042–1050. https://doi.org/10.1139/z97-125
Krutzsch PH (1955) Observations on the Mexican free-tailed bat, Tadarida mexicana. J Mammal 36:236–242
Krutzsch PH (1975) Reproduction of the canyon bat, Pipistrellus hesperus, in southwestern United States. Am J Anat 143:163–200. https://doi.org/10.1002/aja.1001430203
Krutzsch PH (1979) Male reproductive patterns in nonhibernating bats. Reproduction 56:333–344. https://doi.org/10.1530/jrf.0.0560333
Krutzsch PH (2000) Anatomy, physiology and cyclicity of the male reproductive tract. In: Crichton EG, Krutzsch PH (eds) Reproductive Biology of Bats. Academic Press, London, pp 91–155
Krutzsch PH, Crichton EG (1990) Reproductive biology of the male bent-winged bat, Miniopterus schreibersii (Vespertilionidae) in southeast South Australia. Cells Tissues Organs 139:109–125. https://doi.org/10.1159/000146987
Krutzsch PH, Fleming TH, Crichton EG (2002) Reproductive biology of male mexican free-tailed bats (Tadarida Brasiliensis Mexicana). J Mammal 83:489–500. https://doi.org/10.1644/1545-1542(2002)083<0489:RBOMMF>2.0.CO;2
Krutzsch PH, Watson RH, Lox CD (1976) Reproductive biology of the male leaf-nosed bat, Macrotus waterhousii in southwestern United States. Anat Rec 184:611–635. https://doi.org/10.1002/ar.1091840403
Krutzsch PH, Young RA, Crichton EG (1992) Observations on the reproductive-biology and anatomy of Rhinolophus megaphyllus (Chiroptera, Rhinolophidae) in eastern Australia. Aust J Zool 40:533. https://doi.org/10.1071/ZO9920533
Kuramoto T (1977) Mammals of Japan (15) : Order Chiroptera, Genus Rhinolophus. Honyurui Kagaku [Mammalian Science] 35:31–57
Kurohmaru M, Saruwatari T, Kimura J, Mukohyama M, Watanabe G, Taya K, Hayashi Y (2002) Seasonal changes in spermatogenesis of the Japanese lesser horseshoe bat, Rhinolophus cornutus from a morphological viewpoint. Okijimas Folia Anat Jpn 79:93–100
Lee JH (2018) Male reproductive cycle of hibernating Korean greater horseshoe bat, Rhinolophus ferrumequinum korai (Chiroptera: Rhinolophidae): annual cycle of the seminiferous epithelium and morphological changes of the testes. Eur Zool J 85:105–118. https://doi.org/10.1080/24750263.2018.1447029
Lee JH (in press) Vaginal plug formation and release in female hibernating Korean greater horseshoe bat, Rhinolophus ferrumequinum korai (Chiroptera: Rhinolophidae) during the annual reproductive cycle. Zoomorphology. https://doi.org/10.1007/s00435-019-00467-z
Marshall AJ, Corbet PS (1959) The breeding biology of equatorial vertebrates: reproduction of the bat Chaerephon hindei Thomas at latitude 0°26′N. Proc Zool Soc Lond 132:607–616. https://doi.org/10.1111/j.1469-7998.1959.tb05539.x
Martin RE, Pine RH, DeBlase AF (2011) A Manual of Mammalogy: with Keys to Families of the World, 3rd edn. Waveland Press, Long Grove
Martins FF, Beguelini MR, Puga CCI, Morielle-Versute E, Vilamaior PSL, Taboga SR (2016) Morphophysiology and ultrastructure of the male reproductive accessory glands of the bats Carollia perspicillata, Glossophaga soricina and Phyllostomus discolor (Chiroptera: Phyllostomidae). Acta Histochem 118:640–651. https://doi.org/10.1016/j.acthis.2016.07.005
Martins FF, Puga CCI, Beguelini MR, Morielle-Versute E, Vilamaior PSL, Taboga SR (2015) Comparative analysis of the male reproductive accessory glands of bat species from the five Brazilian subfamilies of the family Phyllostomidae (Chiroptera). J Morphol 276:470–480. https://doi.org/10.1002/jmor.20354
Matthews LH (1937) The form of the penis in the British rhinolophid bats, compared with that in some of the vespertilionid bats; and (2) the female sexual cycle in the British horse-shoe bats, Rhinolophus ferrumequinum insulanus Barrett-Hamilton and R. hipposideros minutus. Trans Zool Soc Lond 23:213–223
Matthews LH (1942) Notes on the genitalia and reproduction of some African rats. Proc Zool Soc Lond 111:289–342
Menzies JI (1973) A study of leaf-nosed bats (Hipposideros caffer and Rhinolophus landeri) in a cave in northern Nigeria. J Mammal 54:930–945. https://doi.org/10.2307/1379087
Miotti MD, Mollerach MI, Barquez RM (2018) Anatomy and histology of the prostate and glands of Cowper in three species of Neotropical bats. J Morphol 279:294–301. https://doi.org/10.1002/jmor.20771
Mitchell GC (1965) A natural history study of the funnel-eared bat, Natalus stramineus. M.S. thesis, University of Arizona, Tucson
Mori T, Oh YK, Uchida TA (1982) Sperm storage in the oviduct of the Japanese greater horseshoe bat, Rhinolophus ferrumequinum nippon. J Fac Agric Kyushu Univ 27:47–53
Mutere FA (1973) Reproduction in two species of equatorial free-tailed bats (Molossidae). Afr J Ecol 11:271–280
Negrin AC, Beguelini MR, Puga CCI, Christante CM, Bueno LM, Morielle-Versute E, Vilamaior PSL, Taboga SR (2014) Structure, histochemistry, ultrastructure and seasonal variations of the male prostatic complex in the black Myotis bat, Myotis nigricans (Chiroptera: Vespertilionidae). Reprod Fertil Dev 26:1188. https://doi.org/10.1071/RD13217
Nojiri T, Werneburg I, Son NT, Tu VT, Sasaki T, Maekawa Y, Koyabu D (2018) Prenatal cranial bone development of Thomas’s horseshoe bat (Rhinolophus thomasi): with special reference to petrosal morphology. J Morphol 279:809–827. https://doi.org/10.1002/jmor.20813
Oh YK, Mori T, Uchida TA (1983) Studies on the vaginal plug of the Japanese greater horseshoe bat, Rhinolophus ferrumequinum nippon. J Reprod Fertil 68:365–369. https://doi.org/10.1530/jrf.0.0680365
Oh YK, Mori T, Uchida TA (1985a) Prolonged survival of the Graafian follicle and fertilization in the Japanese greater horsehoe bat, Rhinolophus ferrumequinum nippon. J Reprod Fertil 73:121–126. https://doi.org/10.1530/jrf.0.0730121
Oh Y, Mori T, Uchida TA (1985b) Spermiogenesis in the Japanese greater horseshoe bat, Rhinolophus ferrumequinum nippon. J Fac Agric Kyushu Univ 29:203–209
Oxberry BA (1979) Female reproductive patterns in hibernating bats. Reproduction 56:359–367. https://doi.org/10.1530/jrf.0.0560359
Pal AN (1983) Seasonal changes in the male sex accessory glands of the Indian leaf-nosed bat, Hipposideros speoris (Schneider). Comp Physiol Ecol 8:12–16
Patil DR (1968) Reproduction in the Indian leaf-nosed bat, Hipposideros fulvus fulvus (Gray). Ph.D. thesis, Nagpur University, Nagpur
Pearson OP, Koford MR, Pearson AK (1952) Reproduction of the lump-nosed bat (Corynorhinus rafinesquei) in California. J Mammal 33:273–320. https://doi.org/10.1644/859.1.Key
Pinheiro PFF, Almeida CCD, Segatelli TM, Martinez M, Padovani CR, Martinez FE (2003) Structure of the pelvic and penile urethra-relationship with the ducts of the sex accessory glands of the Mongolian gerbil (Meriones unguiculatus). J Anat 202:431–444. https://doi.org/10.1046/j.1469-7580.2003.00181.x
Price D (1963) Comparative aspects of development and structure in the prostate. Natl Cancer Inst Monogr 12:1–27
Prins GS, Lindgren M (2015) Accessory sex glands in the male. Knobil Neill’s Physiol Reprod 1:773–804
Puga CCI, Beguelini MR, Negrin AC, Christante CM, Morielle-Versute E, Vilamaior PSL, Taboga SR (2012) Structure, histochemistry and ultrastructure of the male reproductive accessory glands in the neotropical flat-faced fruit-eating bat Artibeus planirostris (Chiroptera: Phyllostomidae). Reprod Fertil Dev 25:558–569. https://doi.org/10.1071/RD12029
Racey PA (1979) The prolonged storage and survival of spermatozoa in Chiroptera. J Reprod Fertil 56:391–402. https://doi.org/10.1530/jrf.0.0560391
Racey PA, Tam WH (1974) Reproduction in male Pipistrellus pipistrellus (Mammalia: Chiroptera). J Zool 172:101–122. https://doi.org/10.1111/j.1469-7998.1974.tb04096.x
Rochel SS, Bruni-Cardoso A, Taboga SR, Vilamaior PSL, Góes RM (2007) Lobe identity in the Mongolian gerbil prostatic complex: a new rodent model for prostate study. Anat Rec 290:1233–1247. https://doi.org/10.1002/ar.20585
Rossiter SJ, Gareth J, Ransome RD, M. BE (2000a) Parentage, reproductive success and breeding behaviour in the greater horseshoe bat (Rhinolophus ferrumequinum). Proc R Soc Lond B Biol Sci 267:545–551. https://doi.org/10.1098/rspb.2000.1035
Rossiter SJ, Jones G, Ransome RD, Barratt EM (2000b) Genetic variation and population structure in the endangered greater horseshoe bat Rhinolophus ferrumequinum. Mol Ecol 9:1131–1135. https://doi.org/10.1046/j.1365-294x.2000.00982.x
Sano A (2001) A population study of the Japanese greater horseshoe bat, Rhinolophus ferrumequinum, in the Izumo mines, Ishikawa Prefecture, Japan. Bull Mie Pref Sci Tech Prom Ctr 13:1–68
Santos RTS, Pires LRM, Albernaz ESS, Andrade CS, Santiago CS, Morielle-Versute E, Taboga SR, Beguelini MR (2018) Morphological analysis of the male reproductive accessory glands of the bat Artibeus lituratus (Phyllostomidae: Chiroptera). J Morphol 279:228–241. https://doi.org/10.1002/jmor.20767
Sato Y (2019) Adaptive significance of sperm storage within cauda epididymis during hibernation period in the Japanese little horseshoe bat, Rhinolophus cornutus, having delayed fertilization. Ph.D. thesis, Niigata University, Niigata
Setchell B, Breed W (2006) Anatomy, vasculature, and innervation of the male reproductive tract. In: Neill JD (ed) Physiology of Reproduction. Elsevier, Amsterdam, pp 772–835
Sharifi M, Ghorbani R, Akmali V (2004) Reproductive cycle in Pipistrellus kuhlii (Chiroptera, Vespertilionidae) in western Iran. Mammalia 68:323–327. https://doi.org/10.1515/mamm.2004.031
Singh B (2017) Dyce, Sack and Wensing’s Textbook of Veterinary Anatomy, 5th edn. Elsevier Health Sciences, London
Smith JD (1976) Chiropteran evolution. In: Baker RJ, Jones JK, Carter DC (eds) Biology of Bats of the New World Family Phylostomidae, Part I. Special Publication of the Museum of Texas Technical University, Lubbock, pp 49–69
Springer MS (2013) Phylogenetics: bats united, microbats divided. Curr Biol 23:R999–R1001. https://doi.org/10.1016/j.cub.2013.09.053
Uchida TA (1987) Prolonged storage of spermatozoa in hibernating bats. Recent Adv Study Bats 351–365
van der Merwe M, Stirnemann RL (2007) Reproduction of the banana bat, Neoromicia nanus, in Mpumalanga Province, South Africa, with a discussion on sperm storage and latitudinal effects on reproductive strategies. Afr J Wildl Res 37:53–60. https://doi.org/10.3957/0379-4369-37.1.53
Wilson DE, Mittermeier RA (2019) Handbook of the Mammals of the World. Vol. 9. Bats. Lynx Edicions, Barcelona
Wimsatt WA, Enders AC (1980) Structure and morphogenesis of the uterus, placenta, and paraplacental organs of the Neotropical disc-winged bat Thyroptera tricolor spix (Microchiroptera: Thyropteridae). Am J Anat 159:209–243. https://doi.org/10.1002/aja.1001590208
Yohe LR, Hoffmann S, Curtis A (2018) Vomeronasal and olfactory structures in bats revealed by diceCT clarify genetic evidence of function. Front Neuroanat 12:32. https://doi.org/10.3389/fnana.2018.00032
Acknowledgements
We thank the anonymous reviewer and John R. Wible for improving the manuscript. We thank Masami Fujinoki for helping our field study. We acknowledge financial support from # 9610466 and JSPS #18H04816, #18H02492, #18 K19359, #18KK0207, JRPs-LEAD with DFG, and City University of Hong Kong Start-up Grant to D.K, and Seoul National University Research Grant in 2019 and Basic Science Research Program through the National Research Foundation of Korea NRF-2018R1D1A1B07041621 to J.K.
Author information
Authors and Affiliations
Contributions
J.H.S. collected the data, conceived the results, and wrote the paper. D.F. and J.K. contributed in conceiving the data. T.N, K.M., and J.K. participated in specimen preparation and data collection. D.K. designed the study, prepared the specimens, conceived the results, and wrote the paper.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sohn, J.H., Fukui, D., Nojiri, T. et al. Three-Dimensional and Histological Observations on Male Genital Organs of Greater Horseshoe Bat, Rhinolophus ferrumequinum. J Mammal Evol 28, 559–571 (2021). https://doi.org/10.1007/s10914-020-09525-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10914-020-09525-6