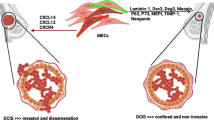
Abstract
The lumens present in ductal structures are required for transport of fluids and air. Studies in model organisms and cells in culture suggest that lumens can be generated by multiple mechanisms including apoptosis of centrally located cells, and re-modeling of epithelia. Several studies point to a role for apoptosis during lumen formation in the mammary ducts. However, a role for other mechanisms during lumen formation in the mammary ducts is largely unexplored. Understanding how lumens are formed and maintained free of cells is of clinical importance because filling of the luminal space is associated with cancer and inflammation. Thus, further investigation can lead to new diagnostic and therapeutic opportunities.


Similar content being viewed by others
Abbreviations
- ERα:
-
estrogen receptor α
- ECM:
-
extracellular matrix
- EGF:
-
epidermal growth factor
- HGF:
-
hepatocyte growth factor
- MEC:
-
mammary epithelial cell
- MMTV:
-
Mouse Mammary Tumor Virus
- TEB:
-
terminal end bud
- 3D:
-
three-dimensional
- TRAIL:
-
Tumor necrosis factor Related Apoptosis Inducing Ligand
- VAC:
-
vacuolar apical compartment
- WAP:
-
whey acidic protein
References
Coleman S, Silberstein GB, Daniel CW. Ductal morphogenesis in the mouse mammary gland: evidence supporting a role for epidermal growth factor. Dev Biol 1988;127(2):304–15.
Kenney NJ, Smith GH, Rosenberg K, Cutler ML, Dickson RB. Induction of ductal morphogenesis and lobular hyperplasia by amphiregulin in the mouse mammary gland. Cell Growth Differ 1996;7(12):1769–81.
Berdichevsky F, Alford D, D’Souza B, Taylor-Papadimitriou J. Branching morphogenesis of human mammary epithelial cells in collagen gels. J Cell Sci 1994;107(Pt 12):3557–68.
Daniel CW, Silberstein GB, Strickland P. Direct action of 17 beta-estradiol on mouse mammary ducts analyzed by sustained release implants and steroid autoradiography. Cancer Res 1987;47(22):6052–7.
Bocchinfuso WP, Lindzey JK, Hewitt SC, Clark JA, Myers PH, Cooper R, et al. Induction of mammary gland development in estrogen receptor-alpha knockout mice. Endocrinology 2000;141(8):2982–94.
Atwood CS, Hovey RC, Glover JP, Chepko G, Ginsburg E, Robison WG, et al. Progesterone induces side-branching of the ductal epithelium in the mammary glands of peripubertal mice. J Endocrinol 2000;167(1):39–52.
Soriano JV, Pepper MS, Nakamura T, Orci L, Montesano R. Hepatocyte growth factor stimulates extensive development of branching duct-like structures by cloned mammary gland epithelial cells. J Cell Sci 1995;108(Pt 2):413–30.
Murtagh J, McArdle E, Gilligan E, Thornton L, Furlong F, Martin F. Organization of mammary epithelial cells into 3D acinar structures requires glucocorticoid and JNK signaling. J Cell Biol 2004;166(1):133–43.
Montesano R, Soulie P. Retinoids induce lumen morphogenesis in mammary epithelial cells. J Cell Sci 2002;115(Pt 23):4419–31.
Sternlicht MD. Key stages in mammary gland development: the cues that regulate ductal branching morphogenesis. Breast Cancer Res 2006;8(1):201.
Fata JE, Werb Z, Bissell MJ. Regulation of mammary gland branching morphogenesis by the extracellular matrix and its remodeling enzymes. Breast Cancer Res 2004;6(1):1–11.
Humphreys RC, Krajewska M, Krnacik S, Jaeger R, Weiher H, Krajewski S, et al. Apoptosis in the terminal endbud of the murine mammary gland: a mechanism of ductal morphogenesis. Development 1996;122(12):4013–22.
Andrechek ER, Hardy WR, Laing MA, Muller WJ. Germ-line expression of an oncogenic erbB2 allele confers resistance to erbB2-induced mammary tumorigenesis. Proc Natl Acad Sci USA 2004;101(14):4984–9.
Frech MS, Halama ED, Tilli MT, Singh B, Gunther EJ, Chodosh LA, et al. Deregulated estrogen receptor alpha expression in mammary epithelial cells of transgenic mice results in the development of ductal carcinoma in situ. Cancer Res 2005;65(3):681–5.
Harris J, Lippman, M., Morrow, M., Osborne, C. Diseases of the Breast. Philadelphia, PA: Williams & Wilkins; 1999.
Hennighausen L, Robinson GW. Information networks in the mammary gland. Nat Rev, Mol Cell Biol 2005;6(9):715–25.
Nelson CM, Bissell MJ. Modeling dynamic reciprocity: engineering three-dimensional culture models of breast architecture, function, and neoplastic transformation. Semin Cancer Biol 2005;15(5):342–52.
Blatchford DR, Quarrie LH, Tonner E, McCarthy C, Flint DJ, Wilde CJ. Influence of microenvironment on mammary epithelial cell survival in primary culture. J Cell Physiol 1999;181(2):304–11.
Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci USA 1992;89(19):9064–8.
Muthuswamy SK, Li D, Lelievre S, Bissell MJ, Brugge JS. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat Cell Biol 2001;3(9):785–92.
Debnath J, Mills K, Collins N, Reginato M, Muthuswamy S, Brugge J. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell 2002;111(1):29.
Lu PJ, Lu QL, Rughetti A, Taylor-Papadimitriou J. bcl-2 overexpression inhibits cell death and promotes the morphogenesis, but not tumorigenesis of human mammary epithelial cells. J Cell Biol 1995;129(5):1363–78.
Reginato MJ, Mills KR, Becker EB, Lynch DK, Bonni A, Muthuswamy SK, et al. Bim regulation of lumen formation in cultured mammary epithelial acini is targeted by oncogenes. Mol Cell Biol 2005;25(11):4591–601.
Heermeier K, Benedict M, Li M, Furth P, Nunez G, Hennighausen L. Bax and Bcl-xs are induced at the onset of apoptosis in involuting mammary epithelial cells. Mech Dev 1996;56(1–2):197–207.
Metcalfe AD, Gilmore A, Klinowska T, Oliver J, Valentijn AJ, Brown R, et al. Developmental regulation of Bcl-2 family protein expression in the involuting mammary gland. J Cell Sci 1999;112(Pt 11):1771–83.
Schorr K, Li M, Bar-Peled U, Lewis A, Heredia A, Lewis B, et al. Gain of Bcl-2 is more potent than bax loss in regulating mammary epithelial cell survival in vivo. Cancer Res 1999;59(11):2541–5.
Li M, Hu J, Heermeier K, Hennighausen L, Furth PA. Apoptosis and remodeling of mammary gland tissue during involution proceeds through p53-independent pathways. Cell Growth Differ 1996;7(1):13–20.
Jerry DJ, Kuperwasser C, Downing SR, Pinkas J, He C, Dickinson E, et al. Delayed involution of the mammary epithelium in BALB/c-p53null mice. Oncogene 1998;17(18):2305–12.
Zhao Y, Tan J, Zhuang L, Jiang X, Liu ET, Yu Q. Inhibitors of histone deacetylases target the Rb-E2F1 pathway for apoptosis induction through activation of proapoptotic protein Bim. Proc Natl Acad Sci USA 2005;102(44):16090–5.
Merlo GR, Basolo F, Fiore L, Duboc L, Hynes NE. p53-dependent and p53-independent activation of apoptosis in mammary epithelial cells reveals a survival function of EGF and insulin. J Cell Biol 1995;128(6):1185–96.
Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol 2001;13(5):555–62.
Coucouvanis E, Martin GR. Signals for death and survival: a two-step mechanism for cavitation in the vertebrate embryo. Cell 1995;83(2):279–87.
Murray P, Edgar D. Regulation of programmed cell death by basement membranes in embryonic development. J Cell Biol 2000;150(5):1215–21.
Reginato MJ, Mills KR, Paulus JK, Lynch DK, Sgroi DC, Debnath J, et al. Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat Cell Biol 2003;5(8):733–40.
Marani M, Hancock D, Lopes R, Tenev T, Downward J, Lemoine NR. Role of Bim in the survival pathway induced by Raf in epithelial cells. Oncogene 2004;23(14):2431–41.
Fukazawa H, Noguchi K, Masumi A, Murakami Y, Uehara Y. BimEL is an important determinant for induction of anoikis sensitivity by mitogen-activated protein/extracellular signal-regulated kinase kinase inhibitors. Molecular Cancer Therapeutics 2004;3(10):1281–8.
Quadros MR, Connelly S, Kari C, Abrams MT, Wickstrom E, Rodeck U. EGFR-dependent downregulation of Bim in epithelial cells requires MAPK and PKC-delta activities. Cancer Biology and Therapy 2006;5(5):498–504.
Yang JM, O’Neill P, Jin W, Foty R, Medina DJ, Xu Z, et al. Extracellular matrix metalloproteinase inducer (CD147) confers resistance of breast cancer cells to Anoikis through inhibition of Bim. J Biol Chem 2006;281(14):9719–27.
Jorgensen K, Skrede M, Cruciani V, Mikalsen SO, Slipicevic A, Florenes VA. Phorbol ester phorbol-12-myristate-13-acetate promotes anchorage-independent growth and survival of melanomas through MEK-independent activation of ERK1/2. Biochem Biophys Res Commun 2005;329(1):266–74.
Gilmore AP, Valentijn AJ, Wang P, Ranger AM, Bundred N, O’Hare MJ, et al. Activation of BAD by therapeutic inhibition of epidermal growth factor receptor and transactivation by insulin-like growth factor receptor. J Biol Chem 2002;277(31):27643–50.
Gilmore AP, Metcalfe AD, Romer LH, Streuli CH. Integrin-mediated survival signals regulate the apoptotic function of Bax through its conformation and subcellular localization. J Cell Biol 2000;149(2):431–46.
Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science 1995;267(5199):891–3.
Pullan S, Wilson J, Metcalfe A, Edwards GM, Goberdhan N, Tilly J, et al. Requirement of basement membrane for the suppression of programmed cell death in mammary epithelium. J Cell Sci 1996;109(Pt 3):631–42.
Li N, Zhang Y, Naylor MJ, Schatzmann F, Maurer F, Wintermantel T, et al. Beta1 integrins regulate mammary gland proliferation and maintain the integrity of mammary alveoli. EMBO J 2005;24(11):1942–53.
Naylor MJ, Li N, Cheung J, Lowe ET, Lambert E, Marlow R, et al. Ablation of beta1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J Cell Biol 2005;171(4):717–28.
White DE, Kurpios NA, Zuo D, Hassell JA, Blaess S, Mueller U, et al. Targeted disruption of beta1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell 2004;6(2):159–70.
Huang J, Hardy JD, Sun Y, Shively JE. Essential role of biliary glycoprotein (CD66a) in morphogenesis of the human mammary epithelial cell line MCF10F. J Cell Sci 1999;112(Pt 23):4193–205.
Kirshner J, Chen CJ, Liu P, Huang J, Shively JE. CEACAM1-4S, a cell–cell adhesion molecule, mediates apoptosis and reverts mammary carcinoma cells to a normal morphogenic phenotype in a 3D culture. Proc Natl Acad Sci USA 2003.
Helminen HJ, Ericsson JL. Studies on mammary gland involution. II. Ultrastructural evidence for auto- and heterophagocytosis. J Ultrastruct Res 1968;25(3):214–27.
Mills KR, Reginato M, Debnath J, Queenan B, Brugge JS. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is required for induction of autophagy during lumen formation in vitro. Proc Natl Acad Sci USA 2004;101(10):3438–43.
Zegers MM, O’Brien LE, Yu W, Datta A, Mostov KE. Epithelial polarity and tubulogenesis in vitro. Trends Cell Biol 2003;13(4):169–76.
Hirai Y, Lochter A, Galosy S, Koshida S, Niwa S, Bissell MJ. Epimorphin functions as a key morphoregulator for mammary epithelial cells. J Cell Biol 1998;140(1):159–69.
Hirai Y, Radisky D, Boudreau R, Simian M, Stevens ME, Oka Y, et al. Epimorphin mediates mammary luminal morphogenesis through control of C/EBPbeta. J Cell Biol 2001;153(4):785–94.
Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005;8(3):241–54.
Lubarsky B, Krasnow MA. Tube morphogenesis: making and shaping biological tubes. Cell 2003;112(1):19–28.
Yu W, O’Brien LE, Wang F, Bourne H, Mostov KE, Zegers MM. Hepatocyte growth factor switches orientation of polarity and mode of movement during morphogenesis of multicellular epithelial structures. Mol Biol Cell 2003;14(2):748–63.
Zahir N, Lakins JN, Russell A, Ming W, Chatterjee C, Rozenberg GI, et al. Autocrine laminin-5 ligates alpha6beta4 integrin and activates RAC and NFkappaB to mediate anchorage-independent survival of mammary tumors. J Cell Biol 2003;163(6):1397–407.
Irie HY, Pearline RV, Grueneberg D, Hsia M, Ravichandran P, Kothari N, et al. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial–mesenchymal transition. J Cell Biol 2005;171(6):1023–34.
Wrobel CN, Debnath J, Lin E, Beausoleil S, Roussel MF, Brugge JS. Autocrine CSF-1R activation promotes Src-dependent disruption of mammary epithelial architecture. J Cell Biol 2004;165(2):263–73.
Acknowledgements
We thank Dr. Danielle K. Carroll (Harvard Medical School) for critical reading of the manuscript. We thank Jim Duffy for assistance with art work. MJR was supported by funds from Drexel University College of Medicine. SKM was supported by CA098830, The V Foundation Scholar award, Rita Allen Scholar award, FACT, Glen Cove C.A.R.E.S, and LIBC Foundation.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Reginato, M.J., Muthuswamy, S.K. Illuminating the Center: Mechanisms Regulating Lumen Formation and Maintenance in Mammary Morphogenesis. J Mammary Gland Biol Neoplasia 11, 205–211 (2006). https://doi.org/10.1007/s10911-006-9030-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10911-006-9030-4