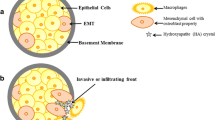
Abstract
Radiographic mammary calcifications occur in 30–50% of breast cancers and constitute one of the most important diagnostic markers of both benign and malignant lesions of the breast. The presence of oxalate-type microcalcification appears to be a reliable criterion in favor of the benign nature of the lesion or, at most, of a lobular carcinoma in situ. In contrast, calcium hydroxyapatite (HA) crystals are associated with both benign and malignant breast tumors. Although the diagnostic value of microcalcifications in breast cancer is of great importance, the genesis of these calcifications is unclear. Despite numerous histological ultrastructure studies of HA deposits in breast carcinomas, to date there have been limited investigations of the potential role of these crystals in breast cancer. We review the literature examining the biological effects of HA crystals in breast cancer cell lines, specifically the mechanism of HA-induced mitogenesis and upregulation of gene expression.
Similar content being viewed by others
Abbreviations
- BSP:
-
bone sialoprotein
- COX:
-
cyclooxygenase
- DCIS:
-
ductal carcinoma in situ
- EGF:
-
epidermal growth factor
- HA:
-
hydroxyapatite
- HFF:
-
human foreskin fibroblasts
- HMEC:
-
human mammary epithelial cells
- IL-1β:
-
interleukin-1β
- MEK:
-
MAP kinase kinase
- MMP:
-
matrix metalloproteinase
- PC:
-
phosphocitrate
- PGE2:
-
prostaglandin E2
- PI3-K:
-
phosphatidylinositol 3-kinase
- PKC:
-
Ȧ Aprotein kinase C
References
Tabar L, Chen HH, Duffy SW, Yen MF, Chiang CF, Dean PB, Smith RA. A novel method for prediction of long-term outcome of women with T1a, T1b, and 10–14 mm invasive breast cancers: A prospective study. Lancet 2000;355:429–33.
Holland R, Peterse J, Millis R. Ductal carcinoma in situ: Aproposal for a new classification. Semin Diagn Pathol 1994;11:167–80.
Pasteris J, Chou I. Fluid-deposited graphitic inclusions in quartz: Comparison between KTB core samples and artifically re-equilibrated natural inclusions. Geochim Cosmochim Acta 1998;62:109–22.
Leborg en R, DE CbB, McClure H. Specimen radiography: a mandatory adjunct to mammography. Am J Clin Pathol 1973;59:782–8.
Castronovo V, Bellahcene A. Evidence that breast cancer associated microcalcifications are mineralized malignant cells. Int J Cancer 1998;12:305–8.
Holme T, Reis M, Thimpson A, Robertson A, Parham D, Hickman P, Preece P. Is mammographic microcalcifiication of biological significance? Eur J Surg Oncol 1993;19:250–3.
Lagios MD, Margolin FR, Westdahl PR, Rose MR. Mammographically detected duct carcinoma in situ. Frequency of local recurrence following tylectomy and prognostic effect of nuclear grade on local recurrence. Cancer 1989;63:618–24.
Frappart L, Boudeulle M, Boumendil J, Lin H, Martinon I, Palayer C, Mallet-Guy Y, Raudrant D, Bremond A, Rochet Y. Structure and composition of microcalcifications in benign and malignant lesions of the breast. Hum Pathol 1984;15:880–9.
Proia A, Brinn N. Identification of calcium oxalate crystals using alizarin red S stain. Arch Pathol Lab Med 1985;109:186–9.
Busin g C, Keppler U, Menges V. Differences in microcalcification in breast tumours. Virchows Arch (Pathol Anat) 1981;393:307–3.
Barth V, Franz E, Scholl A. Microcalcifications in mammary glands. Naturwissenschaften 1977;64:278–9.
Hassler O. Microradiographic investigations of calcifications of the female breast. Cancer 1969;23:1103.
Ahmed A. Calcification in human breast carcinomas: Ultrastructural observations. J Pathol 1975;117:247–51.
Tornos C, Silva E, el-Nagger A, Pritzker K. Calcium oxalate crystals in breast biopsies: The missing microcalcifications. Am J Surg Pathol 1990;14:961–8.
Going J, Anderson T, Crocker P, Levison D. Weddellite calcification in the breast: Eighteen cases with implications for breast cancer screening. Histopathology 1990;16:119–24.
Frouge C, Meunier M, Guinebretiere J, Gilles R, Vanel D, Contesso G, Di Paola R, Blery M. Polyhedral microcalcifications at mammography: Histological correlation with calcium oxalate. Radiology 1993;186:681–4.
Kopans DB. Breast Imaging. 2nd ed. Philadelphia: Lippincott-Raven; 1998.
Muir B, Lamb J, Anderson T, Kirkpatrick A. Microcalcification and its relationship to cancer of the breast: Experience in a screening clinic. Clin Radiol 1983;34:193–200.
Jackson V. Monsees, BS. Evaluation of breast microcalcifications. In Breast Imaging, RCNA. Vol. 33. Philadelphia: W.B. Saunders Co; 1995. p. 1109–21.
Sickles E. Breast calcifications: Mammmographic evaluation. Radiology 1986;160:289–93.
Bird R. Critical pathways in analyzing breast calcifications. Radiographics 1995;15:928–34.
Powell R, McSweeney M, Wilson C. X-ray calcifications as the only basis for breast biopsy. Ann Surg 1983;197:555–9.
Franceschi D, Crowe J, Zollinger R, Duchesneau R, Shenk R, Stefanek G, Shuck J. Biopsy of the breast for mammographically detected lesions. Surg Gynecol Obstet 1990;171:449–55.
Park J, Choi H, Bae S, Lee M, Ahn S, Gong G. Clustering of breast calcifications: Revisited. Clin Radiol 2000;55:114–8.
Brandt G, Bassler R. Pathomorphogenese experimenteller Verkalkungen in der weiblichen Brustdruse. Pathol Anat 1969;348:139–54.
Johannessen J, Sobrinho-Simoes M. The origin and significance of thyroid psammoma bodies. Lan Invest 1980;43:287–96.
Stegner H, Pape C. Beitrag zur Feinstruktur der sogenanntan Mikrokalzifikation in Mammatumoren. Zentralbl Allg Path 1972;115:106–12.
Bellahcene A, Merville M, Castronovo V. Expression of bone sialoprotein, a bone matrix protein, in human breast cancer. Cancer Res 1994;54:2823–6.
Misra R. Calcium and disease: molecular determinants of calcium crystal deposition diseases. Cell Mol Life Sci 2000;57:421–8.
Stary HC. Natural history of calcium deposits in atherosclerosis progression and regression. Z Kardiol 2000;89 Suppl 2:28–35.
Arad Y, Spadaro LA, Goodman K, Newstein D, Guerci AD. Prediction of coronary events with electron beam computed tomography. J Am Coll Cardiol 2000;36:1253–60.
Fitzgerald PJ, Ports TA, Yock PG. Contribution of localized calcium deposits to dissection after angioplasty. An observational study using intravascular ultrasound. Circulation 1992;86:64–70.
Morgan M, Cooke M, Christopherson P, Westfall P, McCarthy G. Calcium hydroxyapatite promotes mitogenesis and matrix metalloproteinase expression in human breast cancer cell lines. Mol Carcinog 2001;32:111–7.
Cooke M, McCarthy M, Sallis J, Morgan M. Phosphocitrate inhibits calcium hydroxyapatite induced mitogenesis and upregulation of matrix metalloproteinase-1, interleukin-1, and cyclooxygenase-2 mRNA in human breast cancer cell lines. Breast Cancer Res Treat 2003;79:253–63.
McCarthy G, Augustine J, Baldwin A, Christopherson P, Cheung H, Westfall P, Scheinman R. Molecular mechanisms of basic calcium phosphate crystal-induced activation of human fibroblasts. J Biol Chem 1998;273:35161–9.
Nelson A, Fingleton B, Rothenberg M, Matrisian L. Matrix metalloproteinases: Biological activity and clinical implications. J Clin Oncol 2000;18:1135–49.
Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell M. Matrix metalloproteinase Stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol 1997;139:1861–72.
McCarthy G, Mitchell P, Cheung H. The mitogenic response to stimulation with basic calcium phosophate crystals is accompanied by induction and secretion of collagenase in human fibroblasts. Arthritis and Rheumatism 1991;34:1021–30.
Brogley M, Cruz M, Cheung H. Basic calcium phosphate crystal induction of collagenase 1 and stromelysin expression is dependent on a p42/44 mitogen-activated protein kinase signal transduction pathway. J Cell Physiol 1999;180:215–24.
Rolland P, Martrin P, Jacquemier J, Rolland A, Toga M. Prostaglandins in human breast cancer: evidence suggesting that an elevated prostaglandin production is a marker of high metastatic potential for neoplastic cells. J Natl Cancer Inst 1980;64:1061–70.
Lui X, Rose D. Differential expression and regulation of cyclooxygenase-1 and -2 in two human breast cancer cell lines. Cancer Res. 1996;56:5125–7.
Lauri D, Bertomeu M, Orr F, Bastida E, Sauder D, Buchanan M. Interleukin-1 increases tumor cell adhesion to endothelial cells through an RGD dependent mechanism: In vitro and in vivo studies. Clin Exp Metastasis 1990;8:27–32.
Verhasselt B, Van Damme J, van Larebeke N, Put W, Bracke M, DePotter C, Mareel M. Interleukin-1 is a motility factor for human breast carcinoma cells in vitro: additive effect with interleukin-6. Eur J Cell Biol. 1992;59:449–57.
Schrey M, Patel K. Prostaglandin E2 production and metabolism in human breast cancer cells and breast fibroblasts. Regulation by inflammatory mediators. Br J Cancer 1995;72:1412–9.
Rutter J, Benbow U, Coon C, Brinckerhoff C. Cell-type specific regulation of human interstitial collagenase-1 Gene expression by Interleukin-1 beta in Human Fibroblasts and BC-8701 Breast Cancer Cells. J Cell Biochem 1997;66:322–36.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morgan, M.P., Cooke, M.M. & McCarthy, G.M. Microcalcifications Associated with Breast Cancer: An Epiphenomenon or Biologically Significant Feature of Selected Tumors?. J Mammary Gland Biol Neoplasia 10, 181–187 (2005). https://doi.org/10.1007/s10911-005-5400-6
Issue Date:
DOI: https://doi.org/10.1007/s10911-005-5400-6